Performance of the Oncuria-Detect bladder cancer test for evaluating patients presenting with haematuria: results from a real-world clinical setting
- PMID: 40533776
- PMCID: PMC12178018
- DOI: 10.1186/s12967-025-06749-z
Performance of the Oncuria-Detect bladder cancer test for evaluating patients presenting with haematuria: results from a real-world clinical setting
Abstract
Background: Bladder cancer is the 9th most diagnosed cancer worldwide with high incidences reported in Europe and the United States. Here, we evaluated the real-world performance of a commercially available multiplex immunoassay (Oncuria-Detect, Nonagen Bioscience Corp, Los Angeles, CA, USA) that detects bladder cancer by simultaneously measuring a panel of 10 protein biomarkers in naturally voided urine samples.
Methods: We tested prospectively collected urine samples from a real-world cohort of 931 patients presenting to five US centres, one European centre and one Japanese centre with haematuria, in addition to 69 patients with either kidney or prostate cancer (disease controls). The algorithm training/refinement set comprised 617 subjects and the test set included 383 subjects. Assay results were collated with patient clinical data and a cancer diagnosis was defined by biopsy and pathology. The prevalence of bladder cancer in the study was 20%.
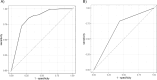
Results: In the training set, the Oncuria-Detect assay correctly identified bladder cancer in 105 of 121 cases. In the test set, the Oncuria-Detect assay correctly identified bladder cancer in 62 of 73 cases resulting in a sensitivity of 85%, a specificity of 72%, and a negative predictive value (NPV) of 95%. The performance of Oncuria was similar for both low-grade/low-stage and high-grade/high-stage.
Conclusions: The multiplex Oncuria assay identified bladder cancer with high sensitivity and NPV. Oncuria's high NPV could effectively rule out 66% of patients from requiring subsequent cystoscopy.
Keywords: Biomarkers; Bladder cancer; Haematuria; Multiplex; Protein; Urinalysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Cedars Sinai Local ethics review board approved. Subject gave written consent. Consent for publication: Not applicable. Competing interests: Dr. Charles Rosser is an officer of Nonagen Bioscience. All other authors declare that they have no competing interests.
Figures
Similar articles
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.Health Technol Assess. 2006 Jun;10(18):iii-iv, xi-259. doi: 10.3310/hta10180. Health Technol Assess. 2006. PMID: 16729917
-
Novel urinary biomarkers for the detection of bladder cancer: A systematic review.Cancer Treat Rev. 2018 Sep;69:39-52. doi: 10.1016/j.ctrv.2018.05.012. Epub 2018 May 29. Cancer Treat Rev. 2018. PMID: 29902678
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
EarlyTect BCD, a Streamlined PENK Methylation Test in Urine DNA, Effectively Detects Bladder Cancer in Patients with Hematuria.J Mol Diagn. 2024 Jul;26(7):613-623. doi: 10.1016/j.jmoldx.2024.04.001. Epub 2024 Apr 25. J Mol Diagn. 2024. PMID: 38677548
References
-
- Bostrom PJ, Bjartell AS, Catto JW, et al. Genomic predictors of outcome in prostate cancer. Eur Urol. 2015;68:1033–44. - PubMed
-
- Van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. - PubMed
-
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical