Brain fluid physiology in ischaemic stroke; more than just oedema
- PMID: 40533800
- PMCID: PMC12175363
- DOI: 10.1186/s12987-025-00671-8
Brain fluid physiology in ischaemic stroke; more than just oedema
Abstract
Background: Cerebrospinal fluid and interstitial fluid dynamics are critical for maintaining homeostasis in the central nervous system. These fluids facilitate waste clearance, micronutrient distribution, and provide a tightly regulated ionic environment. Ischaemic stroke, a leading cause of morbidity and mortality, disrupts this delicate system, compounding the physiological challenges posed by the condition. Despite recent advances in our understanding of the importance of cerebrospinal fluid (CSF) and interstitial fluid (ISF) movement and exchange, the role of this system in stroke pathophysiology remains underexplored.
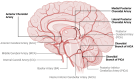
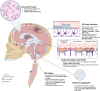
Main body: Emerging evidence indicates that ischaemic stroke acutely alters CSF and ISF movement and exchange, with effects observed at both local and brain-wide levels. In the hyper-acute phase, there is an influx of CSF into perivascular spaces, potentially contributing to early cell swelling. Over time, impaired clearance mechanisms exacerbate ionic and vasogenic oedema, elevating intracranial pressure and further compromising perfusion in the ischaemic penumbra. Mechanistic studies suggest that disruptions in arterial pulsatility, extracellular space microstructure, and aquaporin 4 localisation may underlie these changes. Experimental models have revealed decreased CSF and ISF exchange, movement and outflow in the hours to days following stroke, with implications for waste clearance and secondary injury processes. The interplay between these dynamics and cortical spreading depolarisations, stroke severity, and cerebrovascular physiology adds complexity to understanding the condition's progression.
Conclusion: The disruption of CSF and ISF movement and exchange may represent a significant, yet underappreciated contributor to post-stroke pathology. Addressing these alterations could offer novel therapeutic avenues to mitigate secondary damage, improve central nervous system (CNS) homeostasis, and enhance recovery outcomes. Future research must focus on elucidating the precise mechanisms of CSF and ISF movement and exchange disturbance and exploring targeted interventions to restore normal fluid dynamics in the CNS post-stroke.
Keywords: Cerebral oedema; Cerebrospinal fluid; Cerebrospinal fluid efflux; Glymphatics; Intracranial pressure; Stroke; Stroke pathophysiology.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Cserr HF. Physiology of the choroid plexus. Physiol Rev. 1971;51(2):273–311. - PubMed
-
- Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev. 2013;93(4):1847–92. - PubMed
-
- Rosenberg GA, Kyner WT, Estrada E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am J Physiology-Renal Physiol. 1980;238(1):F42–9. - PubMed
-
- McComb JG, McComb JG, McComb JG. Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg. 1983;59(3):369–83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

