Preclinical evidence of the effect of icariin on diabetic nephropathy: a systematic review and meta-analysis
- PMID: 40533818
- PMCID: PMC12175312
- DOI: 10.1186/s13098-025-01760-2
Preclinical evidence of the effect of icariin on diabetic nephropathy: a systematic review and meta-analysis
Abstract
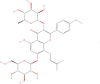
Background: Icariin (ICA), a bioactive flavonoid derived from Epimedium species, has demonstrated anti-inflammatory and anti-fibrotic properties in preclinical studies, suggesting potential therapeutic effects on diabetic nephropathy (DN). However, systematic evaluation of its efficacy remains unclear.
Objective: The purpose of this study is to evaluate the efficacy of Icariin on DN by preclinical evidence and meta-analysis. Meanwhile, the main possible action mechanisms of Icariin against DN were also summarized.
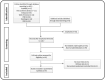
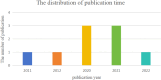
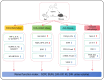
Methods: As of October 1, 2024, we conducted a systematic search across seven prominent Chinese and English databases (CNKI, Wanfang, CBM, PubMed, Cochrane Library, Embase, and Web of Science) to identify studies investigating the therapeutic effects of icariin on DN. PROSPERO has released a summary protocol (registration number: CRD42024564001).
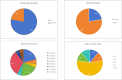
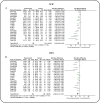
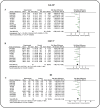
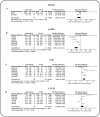
Results: This meta-analysis encompassed nine studies, involving a total of 308 animals, and revealed that icariin significantly reduced blood glucose, SCR, BUN, 24 h UP, 24 h UV, KI, MDA, and IL-1β levels, while augmenting antioxidant enzyme activities (SOD and GPX). Furthermore, ICA lowered TG and TC, indicative of its potential in mitigating risk factors. However, direct comparisons between ICA and angiotensin II receptor blockers (ARB) yielded no statistically significant differences in DN treatment outcomes (p > 0.05). The greatest effects were recorded in high-dose (> 30 mg/kg/day) groups rather than in low-dose (< 30 mg/kg/day) groups. For time-response effects, subgroup analysis indicated that intervention duration of ICA can influence the treatment effect, and more beneficial effects were observed when studies had a drug administration time of < 8 weeks.
Conclusion: Based on an analysis of existing experimental evidence, icariin displays promise in slowing the progression of diabetic nephropathy. To validate its anti-diabetic nephropathy efficacy with greater precision and ensure its readiness for clinical translation, further confirmatory animal studies are warranted.
Keywords: Animal experiment; Diabetic nephropathy; Icariin; Meta-analysis; Systematic review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Effective dose/duration of natural flavonoid quercetin for treatment of diabetic nephropathy: A systematic review and meta-analysis of rodent data.Phytomedicine. 2022 Oct;105:154348. doi: 10.1016/j.phymed.2022.154348. Epub 2022 Jul 19. Phytomedicine. 2022. PMID: 35908521
-
Protective effect of berberine in diabetic nephropathy: A systematic review and meta-analysis revealing the mechanism of action.Pharmacol Res. 2022 Nov;185:106481. doi: 10.1016/j.phrs.2022.106481. Epub 2022 Oct 3. Pharmacol Res. 2022. PMID: 36195307
-
Preclinical Evaluation of Baicalin for the Treatment of Diabetic Nephropathy: A Systematic Review and Meta-Analysis.Planta Med. 2025 Jul 24. doi: 10.1055/a-2615-8249. Online ahead of print. Planta Med. 2025. PMID: 40719085
-
Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta-analyses.Cochrane Database Syst Rev. 2023 Sep 12;9(9):CD015226. doi: 10.1002/14651858.CD015226.pub2. Cochrane Database Syst Rev. 2023. PMID: 37696529 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
-
- Rabbani N, Thornalley PJ. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018;93:803. - PubMed
-
- Gnudi L, Coward RJM, Long DA. Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol Metab. 2016. 10.1016/j.tem.2016.07.002. - PubMed
-
- D. Sharma, P. Bhattacharya, K. Kalia, V. Tiwari. 2017. Diabetic nephropathy new insights into established therapeutic paradigms and novel molecular targets. Diabetes Research and Clinical Practice. 10.1016/j.diabres.2017.04.010 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

