Genomic characterization of antimicrobial resistance and mobile genetic elements in swine gut bacteria isolated from a Canadian research farm
- PMID: 40533851
- PMCID: PMC12175345
- DOI: 10.1186/s42523-025-00432-w
Genomic characterization of antimicrobial resistance and mobile genetic elements in swine gut bacteria isolated from a Canadian research farm
Abstract
Introduction: The widespread use of antimicrobials in the livestock industry has raised global concerns regarding the emergence and spread of antimicrobial resistance genes (ARGs). Comprehensive databases of ARGs specific to different farm animal species can greatly improve the surveillance of ARGs within the agri-food sector and beyond. In particular, defining the association of ARGs with mobile genetic elements (MGEs)-the primary agents responsible for the spread and acquisition of resistant phenotypes among bacterial populations-could help assess the transmissibility potential of clinically relevant ARGs. Recognizing the gut microbiota as a vast reservoir of ARGs, we aimed to generate a representative isolate collection and genome database of the swine gut microbiome, enabling high-resolution characterization of ARGs in relation to bacterial host range and their association with MGEs.
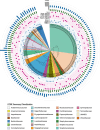
Results: We generated a biobank of bacteria from different sections of the gastrointestinal tracts of four clinically healthy pigs housed at a research farm in Ontario, Canada. The culturing was performed under anaerobic conditions using both selective and general enrichment media to ensure the capture of a diverse range of bacterial families within the swine gut microbiota. We sequenced the genomes of 129 unique isolates encompassing 44 genera and 25 distinct families of the swine gut microbiome. Approximately 85.3% (110 isolates) contained one or more ARGs, with a total of 246 ARGs identified across 38 resistance gene families. Tetracycline and macrolide resistance genes were the most prevalent across different lineages of the swine gut microbiota. Additionally, we observed a wide range of MGEs, including integrative conjugative elements, plasmids, and phages, frequently associated with ARGs, indicating that the swine gut ecosystem is conducive to the horizontal transfer of ARGs. High-throughput alignment of the identified ARG-MGE complexes to large-scale metagenomics datasets of the swine gut microbiome suggests the presence of highly prevalent and conserved resistome sequences across diverse pig populations.
Conclusion: Our findings reveal a highly diverse and relatively conserved reservoir of ARGs and MGEs within the gut microbiome of pigs. A deeper understanding of the microbial host range and potential transmissibility of prevalent ARGs in the swine microbiome can inform development of targeted antimicrobial resistance surveillance and disease control programs.
Keywords: Antimicrobial resistance; Integrative and conjugative elements; Mobile genetic elements; Swine gut microbiome.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: Animal use in this project was approved by the University of Guelph Animal Care Committee (AUP# 3124) and follows Canadian Council of Animal Care guidelines (CCAC, 2009). Consent to participate: Animal use in this project was approved by the University of Guelph Animal Care Committee (AUP# 3124) and follows Canadian Council of Animal Care guidelines (CCAC, 2009). Consent for publication: Not applicable.
Figures



Similar articles
-
Hi-C untangles the temporal dynamics of the children's gut resistome and mobilome, highlighting the role of transposable elements.mBio. 2025 Sep 10;16(9):e0113425. doi: 10.1128/mbio.01134-25. Epub 2025 Aug 12. mBio. 2025. PMID: 40793781 Free PMC article.
-
Impact of doxycycline post-exposure prophylaxis for sexually transmitted infections on the gut microbiome and antimicrobial resistome.Nat Med. 2025 Jan;31(1):207-217. doi: 10.1038/s41591-024-03274-2. Epub 2024 Oct 3. Nat Med. 2025. PMID: 39363100 Free PMC article. Clinical Trial.
-
MGE-associated ARGs exhibit higher expression efficiency than chromosomal non-MGE loci and predominantly contribute to resistance expression in pig farm wastewater.Environ Int. 2025 Aug;202:109653. doi: 10.1016/j.envint.2025.109653. Epub 2025 Jun 30. Environ Int. 2025. PMID: 40618650
-
Prioritization of Critical Factors for Surveillance of the Dissemination of Antibiotic Resistance in Pseudomonas aeruginosa: A Systematic Review.Int J Mol Sci. 2023 Oct 15;24(20):15209. doi: 10.3390/ijms242015209. Int J Mol Sci. 2023. PMID: 37894890 Free PMC article.
-
A meta-analysis of genome-wide association studies to identify candidate genes associated with feed efficiency traits in pigs.J Anim Sci. 2025 Jan 4;103:skaf010. doi: 10.1093/jas/skaf010. J Anim Sci. 2025. PMID: 39847436 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
