Effectiveness of a formulary system on prophylactic oral proton pump inhibitors for drug-induced peptic ulcer in a Japanese tertiary hospital: an interrupted time series analysis
- PMID: 40533872
- PMCID: PMC12175457
- DOI: 10.1186/s40780-025-00459-w
Effectiveness of a formulary system on prophylactic oral proton pump inhibitors for drug-induced peptic ulcer in a Japanese tertiary hospital: an interrupted time series analysis
Abstract
Background: Pharmacy formulary systems have been established recently in various fields of pharmacotherapies. We evaluated the effectiveness of pharmacy formulary interventions on proton pump inhibitors (PPI) used to prevent drug-induced peptic ulcers in a Japanese tertiary hospital.
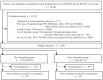
Methods: A retrospective cohort study was conducted in Kurashiki Central Hospital. A pharmacy formulary system of PPIs was implemented on October 1, 2020. Between April 2020 and March 2021, six months before and after the implementation date of the formulary system, inpatients aged ≥ 18 years were included if they newly received drugs (low-dose aspirin, anti-platelets, or non-steroidal anti-inflammatory drugs) recommended for prophylactic PPI use for peptic ulcers within seven days from hospital admission. Eligible patients were divided into two groups based on the implementation date, and changes in PPI prescription patterns were evaluated by interrupted time series analysis, along with the risk of drug-induced peptic ulcers and drug costs.
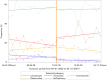
Results: In total, 2,449 inpatients were included. The median age of the pre- and post-formulary group was 60 and 58 years, respectively. The proportion of the targeted PPI prescription increased by 8.75% (95% confidence interval (CI); 0.12, 17.38) in level change, without increased risk of drug-induced peptic ulcers (risk difference -0.41%, 95% CI; -1.38, 0.55). The distribution of medication days in the two groups was similar, and $1,000 per 90 patient days was saved on drug costs.
Conclusion: The formulary system on oral PPIs in a Japanese tertiary hospital contributed to a positive level change in the prescription patterns, without increased risk of drug-induced peptic ulcers. Although slight, the drug costs were saved.
Keywords: Cost-analysis; Drug-induced peptic ulcer; Hospital formulary; Interrupted time series; Proton pump inhibitor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Because the data were used after anonymization, this study was conducted following information disclosure and an opportunity to withdraw by the opt-out method specified in the Japanese ethical guidelines for medical and biological research involving human subjects as of 2021. This study protocol was approved by the Ethics Committee of Kurashiki Central Hospital (No. 3759). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Hashim S, Gomes T, Juurlink D, Hellings C, Mamdani M. The rise and fall of the Thiazolidinediones: impact of clinical evidence publication and formulary change on the prescription incidence of Thiazolidinediones. J Popul Ther Clin Pharmacol. 2013;20(3):e238–242. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

