A Novel Method for Culturing Telencephalic Neurons in Axolotls
- PMID: 40533890
- PMCID: PMC12177116
- DOI: 10.1002/cne.70066
A Novel Method for Culturing Telencephalic Neurons in Axolotls
Abstract
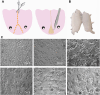
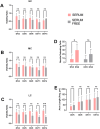
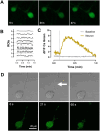
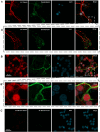
The axolotl (Ambystoma mexicanum), a neotenic salamander with remarkable regenerative capabilities, serves as a key model for studying nervous system regeneration. Despite its potential, the cellular and molecular mechanisms underlying this regenerative capacity remain poorly understood, partly due to the lack of reliable in vitro models for axolotl neural cells. In this study, we developed a novel protocol for primary cultures of adult axolotl telencephalon/pallium, enabling the maintenance of viable and functionally active neural cells. Using calcium imaging and immunocytochemistry, we demonstrated the presence of neuronal and glial markers, synaptic connections, and spontaneous calcium activity, highlighting the functional integrity of the cultured cells. Our findings reveal that these cultures can be maintained in both serum and serum-free conditions, with neurons exhibiting robust neurite outgrowth and responsiveness to injury. This protocol addresses a critical gap in axolotl research by providing a controlled in vitro system to study neurogenesis and regeneration. By offering insights into the regenerative mechanisms of axolotl neurons, this work lays the foundation for comparative studies with mammalian systems, potentially informing therapeutic strategies for neurodegenerative diseases and CNS injuries in humans.
Keywords: axolotl; primary neuron culture; regeneration; telencephalon.
© 2025 The Author(s). The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The Genetic Odyssey of Axolotl Regeneration: Insights and Innovations.Int J Dev Biol. 2024 Dec 12;68(3):103-116. doi: 10.1387/ijdb.240111yl. Int J Dev Biol. 2024. PMID: 39723527 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Development of a Marmoset Apparatus for Automated Pulling to study cooperative behaviors.Elife. 2024 Oct 28;13:RP97088. doi: 10.7554/eLife.97088. Elife. 2024. PMID: 39466838 Free PMC article.
-
A Hybrid 2D/3D Approach for Neural Differentiation Into Telencephalic Organoids and Efficient Modulation of FGF8 Signaling.Bio Protoc. 2025 Jun 20;15(12):e5354. doi: 10.21769/BioProtoc.5354. eCollection 2025 Jun 20. Bio Protoc. 2025. PMID: 40620811 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Aladağ, Z. , Vatandaşlar E., Vilain S., Öztürk G., et al. 2024. “Development of a Robust Gel‐Free 3D Culture System for Generating Spheroids From Axolotl Blastema Cells.” BioRxiv 2024.08.29.610223.
-
- Albert, P. , and Boilly B.. 1988. “Effect of Transferrin on Amphibian Limb Regeneration: A Blastema Cell Culture Study.” Roux's Archives of Developmental Biology 197: 193–196. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

