Evaluation of One-Stage Assays for the Monitoring of Recombinant Human Factor IX Padua Activity After Etranacogene Dezaparvovec Gene Therapy
- PMID: 40534246
- PMCID: PMC12311899
- DOI: 10.1111/hae.70053
Evaluation of One-Stage Assays for the Monitoring of Recombinant Human Factor IX Padua Activity After Etranacogene Dezaparvovec Gene Therapy
Abstract
Introduction: Accurate and reproducible measures of factor activity are required to guide clinical decision-making following gene therapy for haemophilia B (HB). Highly significant discrepancies have been observed in measurements of various factor IX (FIX) concentrates that carry molecular modifications to extend their half-life, arguing for the need for careful analysis of new HB treatment modalities with respect to FIX assay performance.
Aim: To further characterise variability in FIX activity measured using different one-stage assays (OSAs) and chromogenic assays (CAs) in patients with HB receiving gene therapy utilising the FIX Padua variant and to assess whether assay differences were due to the FIX-Padua variant.
Methods: FIX activity was assessed centrally (OSA and CA) and locally (OSA only) using plasma samples collected from a phase 2b and phase 3 study of etranacogene dezaparvovec and in an in vitro study of wild-type (wt) recombinant human FIX (rhFIX) and rhFIX-Padua.
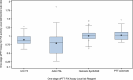
Results: Lower CA than OSA FIX activity for plasma samples from the phase 3 trial was observed (CA:OSA ratio: 0.408 [±0.049]-0.547 [±0.062]). Local OSA:central OSA FIX activity ratios were 0.789 (±0.314)-1.021 (±0.159). Local OSA:central OSA FIX activity ratios across methods and/or reagents were 0.81 (±0.02)-1.28 (±0.04) for rhFIX-wt-spiked samples and 0.67 (±0.02)-1.13 (±0.09) for rhFIX-Padua-spiked samples.
Conclusion: FIX activity differences between central and local OSAs were modest; similar differences were observed in vitro with rhFIX-wt versus rhFIX-Padua. Commonly available OSAs can be used to monitor patients post-etranacogene dezaparvovec administration; we recommend using the same assay platform throughout the post-treatment period.
Keywords: chromogenic assay; factor IX; gene therapy; haemophilia B; one‐stage assay.
© 2025 The Author(s). Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
J.A.: Grant/research support from SOBI, Bayer, Takeda/Shire and CSL Behring; honorarium as member of advisory boards and speaker for BioMarin, Pfizer, Sparks, uniQure, CSL Behring, SOBI, Sanofi, Novo Nordisk, Bayer, Roche, Takeda/Shire and Octapharma. W.M.: Grant/research support from Bayer, Biotest, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Takeda/Shire; consultation/speaker fees from Bayer, Biomarin, Biotest, CSL Behring, Chugai, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, Takeda/Shire; and consultation fees from Bayer, Biomarin, Biotest, CSL Behring, Chugai, Freeline, LFB, Novo Nordisk, Octapharma, Pfizer, Regeneron, Roche, Sanofi, Sobi, Takeda/Shire, and uniQure. M.C.: has received financial support for research from Anthos, Bayer, CSL Behring, Novo Nordisk, and Hoffmann‐La Roche; and honoraria for lecturing or consultancy from Alexion, CSL Behring, Daiichi Sankyo, Sanofi, Spark Therapeutics, Octapharma, Pfizer, Sobi, and Viatris. All funds were received by his institution. Non‐financial conflicts of interest: member of the gene therapy working group of the European Association for Haemophilia and Allied Disorders (EAHAD); and member of the European Reference Network (ERN) EuroBloodNet. S.G., J.T., R.D., S.V.: Employees of uniQure biopharma B.V. at the time of this research; RD is now an employee of Tempuro Bio. P.E.M., B.M.E., S.N., N.G.: Employees of CSL Behring. G.Y.: has received consulting fees from ASC Biotherapeutics, BioMarin, Centessa, CSL Behring, Genentech/Roche, Hema Biologics/LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi Genzyme, Spark, and Takeda, and funds for research support from Sanofi.
Figures


Similar articles
-
Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B.N Engl J Med. 2023 Feb 23;388(8):706-718. doi: 10.1056/NEJMoa2211644. N Engl J Med. 2023. PMID: 36812434 Clinical Trial.
-
Completion of phase 2b trial of etranacogene dezaparvovec gene therapy in patients with hemophilia B over 5 years.Blood Adv. 2025 Jul 22;9(14):3543-3552. doi: 10.1182/bloodadvances.2024015291. Blood Adv. 2025. PMID: 40188458 Free PMC article. Clinical Trial.
-
Gene-ius at work: Hemophilia B treatment enters a new era.Am J Health Syst Pharm. 2025 Sep 9;82(18):960-969. doi: 10.1093/ajhp/zxaf005. Am J Health Syst Pharm. 2025. PMID: 39868419 Review.
-
Factor IX assay discrepancies in the setting of liver gene therapy using a hyperfunctional variant factor IX-Padua.J Thromb Haemost. 2021 May;19(5):1212-1218. doi: 10.1111/jth.15281. Epub 2021 Mar 28. J Thromb Haemost. 2021. PMID: 33636038 Free PMC article.
-
Etranacogene dezaparvovec-drlb gene therapy for patients with hemophilia B (congenital factor IX deficiency).Expert Opin Biol Ther. 2023 Jul-Dec;23(12):1173-1184. doi: 10.1080/14712598.2023.2282138. Epub 2023 Dec 28. Expert Opin Biol Ther. 2023. PMID: 37962325 Review.
References
-
- Iacobucci G., “NICE Recommends New Gene Therapy for Adults With Haemophilia B,” BMJ 385 (2024): q1440. - PubMed
-
- Nature . EU Gets First Hemophilia B Gene Therapy. Nature Biotechnology 2023;41(4):438–438. - PubMed
-
- Sekayan T., Simmons D. H., and von Drygalski A., “Etranacogene Dezaparvovec‐drlb Gene Therapy for Patients With Hemophilia B (Congenital Factor IX Deficiency),” Expert Opinion on Biological Therapy 23, no. 12 (2023): 1173–1184. - PubMed
-
- Dhillon S., “Fidanacogene Elaparvovec: First Approval,” Drugs 84, no. 4 (2024): 479–486. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

