TSUBASA Study: Evaluating Association of Physical Activity and Bleeding Events in People With Haemophilia A Without Factor VIII Inhibitors Receiving Emicizumab
- PMID: 40534253
- PMCID: PMC12311898
- DOI: 10.1111/hae.70070
TSUBASA Study: Evaluating Association of Physical Activity and Bleeding Events in People With Haemophilia A Without Factor VIII Inhibitors Receiving Emicizumab
Abstract
Background: Emicizumab is approved for routine prophylaxis, but limited data exist investigating the relationship between bleeds and exercise in people with haemophilia A (PwHA) receiving emicizumab.
Aim: To evaluate the relationship between physical activity and bleeding outcomes in Japanese PwHA receiving emicizumab prophylaxis.
Methods: This prospective, multicentre, observational study was conducted in Japanese PwHA initiating emicizumab between November 2019 and October 2021. Physical activity and bleeding events were reported using an electronic patient-reported outcomes application, while activity intensity was collected by a wearable activity tracker worn over five 8-day monitoring periods. Data on safety were recorded by investigators using electronic case report forms. Quality of life data were also collected and will be reported in a separate publication.
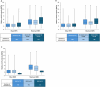
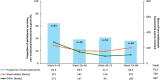
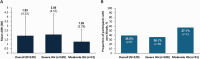
Results: Overall, 129 participants enrolled. Median age was 32 years (range 0-73), and 83.7% of participants had severe HA. Overall, 73 participants performed 968 exercise events, with 18.8% and 6.0% of events classified as moderate and high risk, respectively. Two (0.2%) exercise events were associated with bleeding: one basketball (moderate risk) and one fishing (low risk). In total, 137 adverse events (AEs) were reported in 62 participants. No serious AEs were emicizumab-related. The mean annualised treated bleed rate was 1.9 and 51 (39.5%) participants experienced zero bleeds during the study period (97 weeks).
Conclusion: This study suggests that PwHA receiving emicizumab prophylaxis can participate in a wide range of physical activities with minimal bleeding risk, which supports their access to the health benefits of exercise. Emicizumab remains well tolerated.
Keywords: Japan; emicizumab; exercise; haemophilia A; haemorrhage; physical activity; prospective studies.
© 2025 The Author(s). Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
Kagehiro Amano has received consultancy fees from Chugai Pharmaceutical Co., Ltd; research funding from KM Biologics Co., Ltd; speaker's bureau from Chugai Pharmaceutical Co., Ltd, Sanofi S.A., Bayer AG, Takeda Pharmaceuticals Co., Ltd, Novo Nordisk A/S, CSL Behring, KM Biologics Co., Ltd, Pfizer Inc., Japan Blood Products Organization, Fujimoto Pharmaceutical Co., Ltd and is listed as an entity's board of directors or advisory committee member for Chugai Pharmaceutical Co., Ltd and CSL Behring. Teruhisa Fujii has received consultancy fees from Chugai Pharmaceutical Co., Ltd, Novo Nordisk A/S, Sanofi S.A., Takeda Pharmaceuticals Co., Ltd; speaker's bureau from Chugai Pharmaceutical Co., Ltd, Novo Nordisk A/S, Sanofi S.A., Takeda Pharmaceuticals Co., Ltd, CSL Behring, Fujimoto Pharmaceutical Co., Ltd, Bayer AG, JB Pharma, JMB Pharmaceuticals, ViiV Healthcare, Gilead Sciences, Inc.; research funding from Chugai Pharmaceutical Co., Ltd and is listed as an entity's board of directors or advisory committee member for Novo Nordisk A/S. Akihiro Sawada honoraria: Chugai Pharmaceutical Co., Ltd, Takeda Pharmaceutical Co., Ltd, Sanofi K.K., Novo Nordisk A/S, CSL Behring K.K., Fujimoto Pharmaceutical Co., Ltd and KM Biologics Co., Ltd. Azusa Nagao has received consultancy fees from Chugai Pharmaceutical Co., Ltd and KM Biologics Co., Ltd; research funding from Bayer Yakuhin Ltd, Pfizer Japan Inc., Chugai Pharmaceutical Co., Ltd; honoraria from Sanofi K.K., Takeda Pharmaceuticals Co., Ltd, Chugai Pharmaceutical Co., Ltd, Bayer Yakuhin Ltd, Fujimoto Pharmaceutical Co., Ltd, KM Biologics Co., Ltd, Pfizer Japan Inc., Novo Nordisk A/S, CSL Behring and is listed as an entity's board of directors or advisory committee member for the Japanese Society of Thrombosis and Haemostasis and the International Society of Thrombosis and Haemostasis. Chiai Nagae has received honoraria from Chugai Pharmaceutical Co., Ltd, Novo Nordisk A/S, Sanofi S.A. and Takeda Pharmaceuticals Co., Ltd. Masanori Nojima has received consultancy fees from Chugai Pharmaceutical Co., Ltd and honoraria from EA Pharma Co., Ltd. Nobuaki Suzuki has received honoraria from Chugai Pharmaceutical Co., Ltd, Takeda Pharmaceuticals Co., Ltd, Sanofi S.A., Novo Nordisk A/S, CSL Behring, Bayer Yakuhin Ltd, Pfizer Inc., KM Biologics Co., Ltd and Japan Blood Products Organization. Mika Kawano, Tomomi Shimura, Yoshimasa Sugao and Naoto Hattori are employees and shareholders of Chugai Pharmaceutical Co., Ltd. Keiji Nogami has received research funding and honoraria from Chugai Pharmaceutical Co., Ltd, Sysmex Co., Takeda Pharmaceuticals Co., Ltd, Sanofi S.A., CSL Behring, KM Biologics Co., Ltd, Novo Nordisk A/S, Bayer AG, Fujimoto Pharmaceutical Co., Ltd and Sekisui Medical Co., Ltd.
Figures
References
-
- Fromme A., Dreeskamp K., Pollmann H., Thorwesten L., Mooren F. C., and Völker K., “Participation in Sports and Physical Activity of Haemophilia Patients,” Haemophilia 13, no. 3 (2007): 323–327. - PubMed
-
- Souza J. C., Simoes H. G., Campbell C. S., Pontes F. L., Boullosa D. A., and Prestes J., “Haemophilia and Exercise,” International Journal of Sports Medicine 33, no. 2 (2012): 83–88. - PubMed
-
- Srivastava A., Santagostino E., Dougall A., et al., “WFH Guidelines for the Management of Hemophilia, 3rd Edition,” Haemophilia 26, no. suppl. 6 (2020): 1–158. - PubMed
-
- Wang M., Álvarez‐Román M. T., Chowdary P., Quon D. V., and Schafer K., “Physical Activity in Individuals With Haemophilia and Experience With Recombinant Factor VIII Fc Fusion Protein and Recombinant Factor IX Fc Fusion Protein for the Treatment of Active Patients: A Literature Review and Case Reports,” Blood Coagulation & Fibrinolysis 27, no. 7 (2016): 737–744. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

