A promising frontier of circulating messenger RNA in liquid biopsy: From mechanisms to clinical applications
- PMID: 40534429
- PMCID: PMC12375855
- DOI: 10.1002/ijc.35523
A promising frontier of circulating messenger RNA in liquid biopsy: From mechanisms to clinical applications
Abstract
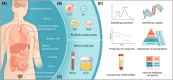
Liquid biopsy can revolutionize cancer patient management as repeated sampling allows real-time monitoring of disease progression and response to treatment. The circulating transcriptome represents a rich source of potential cancer biomarkers, including coding and non-coding RNAs. As techniques that have enabled us to analyze transcriptome profiling become ever more sophisticated, the great potential of extracellular messenger RNA (mRNA) and intracellular (cellular level) mRNA in liquid biopsy has gradually been realized. In this review, we aim to conclude the mRNA-based liquid biopsy in the tumorous context by illustrating the origins of mRNA markers and scientific findings, and summarizing the advantages of mRNAs over DNA-based liquid biopsy. Besides, we give a glimpse of the application of this strategy in clinical studies and analyze the gap between the bench and bedside. We speculate that mRNAs are a promising frontier of liquid biopsy.
Keywords: cell‐free RNA; circulating tumor cell; extracellular vesicle; liquid biopsy; messenger RNA.
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Circulating non-coding RNAs as a tool for liquid biopsy in solid tumors.Epigenomics. 2025 Apr;17(5):335-358. doi: 10.1080/17501911.2025.2467021. Epub 2025 Mar 5. Epigenomics. 2025. PMID: 40040488 Review.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Liquid biopsy - a narrative review with an update on current US governmental clinical trials targeting immunotherapy.Future Sci OA. 2025 Dec;11(1):2527598. doi: 10.1080/20565623.2025.2527598. Epub 2025 Aug 7. Future Sci OA. 2025. PMID: 40772765 Free PMC article. Review.
-
Lessons (to be) learned from liquid biopsies: assessment of circulating cells and cell-free DNA in cancer and pregnancy-acquired microchimerism.Semin Immunopathol. 2025 Feb 1;47(1):14. doi: 10.1007/s00281-025-01042-z. Semin Immunopathol. 2025. PMID: 39893314 Free PMC article. Review.
-
Characterization of Plasma Cell-Free DNA Variants as of Tumor or Clonal Hematopoiesis Origin in 16,812 Advanced Cancer Patients.Clin Cancer Res. 2025 Jul 1;31(13):2710-2718. doi: 10.1158/1078-0432.CCR-24-3335. Clin Cancer Res. 2025. PMID: 39932457 Free PMC article.
References
-
- Pal A, Shinde R, Miralles MS, Workman P, de Bono J. Applications of liquid biopsy in the Pharmacological Audit Trail for anticancer drug development. Nat Rev Clin Oncol. 2021;18(7):454‐467. - PubMed
-
- Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic—implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18(5):297‐312. - PubMed
-
- Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics‐driven oncology. Nat Rev Genet. 2019;20(2):71‐88. - PubMed
-
- Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14(9):531‐548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

