PiggyBac transposase-mediated inducible trophoblast-specific knockdown of Mtor decreases placental nutrient transport and fetal growth
- PMID: 40534504
- PMCID: PMC12409993
- DOI: 10.1042/CS20243293
PiggyBac transposase-mediated inducible trophoblast-specific knockdown of Mtor decreases placental nutrient transport and fetal growth
Abstract
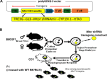
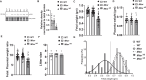
Abnormal fetal growth is associated with perinatal complications and adult disease. The placental mechanistic target of rapamycin (mTOR) signaling activity is positively correlated with placental nutrient transport and fetal growth. However, if this association represents a mechanistic link, it remains unknown. We hypothesized that trophoblast-specific Mtor knockdown in late pregnant mice decreases trophoblast nutrient transport and inhibits fetal growth. PiggyBac transposase-enhanced pronuclear injection was performed to generate transgenic mice containing a trophoblast-specific Cyp19I.1 promoter-driven, doxycycline-inducible luciferase reporter transgene with a Mtor shRNAmir sequence in its 3' untranslated region (UTR). We induced Mtor knockdown by administration of doxycycline starting at E14.5. Dams were killed at E 17.5, and trophoblastspecific gene targeting was confirmed. Placental mTOR protein expression was reduced in these animals, which was associated with a marked inhibition of mTORC1 and mTORC2 signaling activity. Moreover, we observed a decreased expression of System A amino acid transporter isoform SNAT2 and the System L amino acid transporter isoform LAT1 in isolated trophoblast plasma membranes and lower fetal, placental weight, and fetal:placental weight ratio. We also silence the MTOR in cultured primary human trophoblast cells, which inhibited the mTORC1 and C2 signaling, System A and System L amino acid transport activity, and markedly decreased the trafficking of LAT1 and SNAT2 to the plasma membrane. Inhibition of trophoblast mTOR signaling in late pregnancy is mechanistically linked to decreased placental nutrient transport and reduced fetal growth. Modulating trophoblast mTOR signaling may represent a novel intervention in pregnancies with abnormal fetal growth.
Keywords: FGR; fetal development; maternal–fetal exchangeMaternal-Fetal Exchange; mechanistic target of rapamycin; placenta.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

