Investigation of Poststroke Depression Following a Nucleus Accumbens Infarct in Mice
- PMID: 40534558
- PMCID: PMC12372732
- DOI: 10.1161/STROKEAHA.125.050839
Investigation of Poststroke Depression Following a Nucleus Accumbens Infarct in Mice
Abstract
Background: Poststroke depression (PSD) affects ≈33% of individuals 1 year after a stroke. Blood-brain barrier (BBB) dysfunction in the nucleus accumbens (NAc), a hub for emotional processing, reward, and mood regulation, has been linked to stress-induced depressive-like behaviors in male mice. Neurovascular alterations were also observed in postmortem tissue samples from men with a diagnosis of major depression. Thus, we aimed to investigate if BBB changes in the NAc could contribute to PSD pathophysiology.
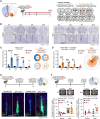
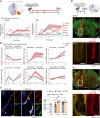
Methods: Stereotaxic injection of ET-1 (endothelin-1), a potent vasoconstrictor, was performed in the NAc of male mice to create a focal brain stroke, and then, infarct size and localization were assessed and quantified. We subsequently evaluated transcriptomic and morphological effects of the infarct on BBB-related genes and cells in the NAc, particularly those known to be altered after stress exposure in mice or human depression. BBB integrity was assessed with a dextran dye, and magnetic resonance imaging scans were conducted before versus after the injection of Gadovist, a contrast agent. Last, a battery of behavioral tests related to depressive- and anxiety-like behaviors was performed to determine if an infarct in the NAc is sufficient to induce a PSD-like phenotype.
Results: Following ET-1 injection, ≈50% of the total lesion was observed in the NAc leading to BBB hyperpermeability in this brain area. BBB gene expression was impacted by ET-1, and also surgery alone and profiles were differentially regulated throughout time up to 14 days. Gliosis in the NAc was observed with increased reactivity of astrocytes and microglia. The effect of ET-1 on PSD-like symptoms was limited. However, body weight, sociability, and activity were affected by surgery with a more pronounced impact of ET-1 on social interactions compared with naive animals.
Conclusions: While no clear PSD phenotype was observed following an ET-1-induced stroke in the NAc of male mice, our study shed light on the technical complexity of focal lesions in deep brain structures, an understudied phenomenon occurring in humans. We provide technical insights for the development of a mouse model of deep brain lesions, characterize its impact at molecular, cellular, and behavioral levels, and highlight the need to control for vascular alterations when performing stroke surgeries.
Keywords: behavior; blood-brain barrier; depression; disease models, animal; gene expression.
Conflict of interest statement
None.
Figures

References
-
- Campbell BCV, Khatri P. Stroke. Lancet. 2020;396:129–142. doi: 10.1016/S0140-6736(20)31179-X - PubMed
-
- Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70. doi: 10.1038/s41572-019-0118-8 - PubMed
-
- Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9:1017–1025. doi: 10.1111/ijs.12357 - PubMed
-
- Taylor-Rowan M, Momoh O, Ayerbe L, Evans JJ, Stott DJ, Quinn TJ. Prevalence of pre-stroke depression and its association with post-stroke depression: a systematic review and meta-analysis. Psychol Med. 2019;49:685–696. doi: 10.1017/S0033291718002003 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

