Enzymatic approaches to nicotinic acid synthesis: recent advances and future prospects
- PMID: 40534613
- PMCID: PMC12174410
- DOI: 10.3389/fbioe.2025.1585736
Enzymatic approaches to nicotinic acid synthesis: recent advances and future prospects
Abstract
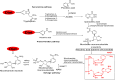
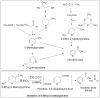
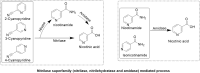
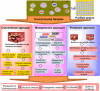
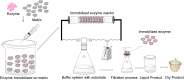
Biocatalyst-mediated reactions have led to revolutionary transformations in the organic synthesis of pharmaceuticals, drugs, and other chemicals. Nicotinic acid (vitamin B3) is an essential precursor for nicotinamide adenine dinucleotide (NAD+) biosynthesis and is vital for numerous metabolic processes. Since the human body cannot synthesize nicotinic acid, it relies on external sources. Therefore, nicotinic acid synthesis has gained huge attraction. In recent years, the industrial production of nicotinic acid has increasingly shifted from traditional chemical methods to more biocatalytic processes, leveraging the power of biocatalysts. This review highlights the biocatalyst-mediated synthesis of nicotinic-acid- and nitrile-metabolizing enzymes through state-of-the-art omics-based techniques to improve enzyme catalytic efficiency and stability via various approaches. Future research prospects and challenges associated with nicotinic acid production are also discussed.
Keywords: biocatalysis; biotransformation; immobilization; nitrilase; omics technology.
Copyright © 2025 Singh, Kalia, Sambyal, Singh, Kumar and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Almatawah Q. A., Cowan D. A. (1999). Thermostable nitrilase catalysed production of nicotinic acid from 3-cyanopyridine. Enzyme Microb. Technol. 25 (8-9), 718–724. 10.1016/S0141-0229(99)00104-0 - DOI
-
- Badoei-Dalfard A., Karami Z., Ramezani-pour N. (2016). Bench scale production of nicotinic acid using a newly isolated Stenotrophomonas maltophilia AC21 producing highly-inducible and versatile nitrilase. J. Mol. Catal. B Enzym 133, S552–S559. 10.1016/j.molcatb.2016.11.019 - DOI
Publication types
LinkOut - more resources
Full Text Sources

