Domain-specific cognitive impairment is differentially affected by Alzheimer disease tau pathologic burden and spread
- PMID: 40534622
- PMCID: PMC12176422
- DOI: 10.1162/imag_a_00405
Domain-specific cognitive impairment is differentially affected by Alzheimer disease tau pathologic burden and spread
Abstract
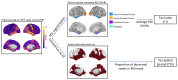
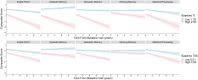
Tau pathology in Alzheimer disease (AD) is often evaluated in regions associated with episodic memory impairment. However, heterogeneous spreading patterns of tau are observed and correspond to impairment in different cognitive domains. We have previously developed a metric to quantify tau spread extent that is robustly sensitive to atypical spreading patterns. Here, we evaluate tau spread relative to domain-specific and general cognitive impairments during early stages of AD. In total, 529 participants with baseline tau positron emission tomography (PET) and neuropsychological testing were separated into disease-stage groups based on amyloid PET positivity and clinical status via Clinical Dementia Rating® (CDR®). General cognition was assessed using the Knight Preclinical Alzheimer Cognitive Composite (Knight PACC). Domain-specific composites were calculated for episodic memory, semantic memory, working memory, and attention/processing speed. Baseline tau burden, the average tau intensity across previously defined AD signature regions, and baseline tau spread extent, the proportion of the brain with elevated tau pathology, were quantified for each participant as Tau Index and Tau Spatial Spread, respectively. Tau burden and tau spread were evaluated relative to baseline and longitudinal cognitive performance, as well as longitudinal clinical progression. Tau burden and tau spread extent both significantly correlate with cognitive impairment in symptomatic AD. Tau burden is most strongly correlated with episodic (r = -0.37, p = 0.02) and semantic (r = -0.36, p = 0.02) memory. In contrast, tau spread extent is most strongly correlated with the Knight PACC (r = -0.37, p = 0.01) and attention/processing speed (r = -0.44, p < 0.01), especially in preclinical AD (r = -0.27, p < 0.01). Tau burden captures more variance than tau spread extent in longitudinal change in the Knight PACC, episodic memory, semantic memory, attention/processing speed, and clinical progression. Tau burden strongly relates to baseline episodic and semantic memory, which may reflect that it is heavily weighted by entorhinal tau, a region previously linked to memory processing. In contrast, stronger associations between tau spread extent and baseline attention/processing speed could reflect the inclusion of additional brain regions, particularly the frontal lobe, which support a wider range of cognitive processing. Additionally, tau spread extent is generally more sensitive to baseline preclinical deficits; however, tau burden better estimates future decline across all cognitive domains and clinical symptom onset. Together, these findings suggest complementary utility of evaluating both tau burden and tau spread extent in early AD progression.
Keywords: Alzheimer disease; Positron Emission Tomography (PET); Tau spread; cognitive domains; cognitive impairment.
Conflict of interest statement
DECLARATION OF COMPETING INTEREST Data collection and sharing for this project were supported by the Dominantly Inherited Alzheimer Network (DIAN, U19-AG032438) funded by the National Institute on Aging (NIA), the Alzheimer’s Association (SG-20–690363-DIAN), the German Center for Neurodegenerative Diseases (DZNE), the Queen Square Dementia Biomedical Research Centre and the Medical Research Council Dementias Platform UK (MR/L023784/1 and MR/009076/1). Partial support has also been provided by research and development grants for dementia from the Japan Agency for Medical Research and Development (JP22dk0207049), AMED, the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), Korea Dementia Research Center (KDRC) funded by the Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea (HI21C0066), the Spanish Institute of Health Carlos III (ISCIII), the Canadian Institutes of Health Research (TAD-125697), the Canadian Consortium of Neurodegeneration and Aging, the Brain Canada Foundation, Fonds de Recherche du Québec, and the Raul Carrea Institute for Neurological Research (FLENI). In addition to the acknowledged funding sources for this research, the authors of this manuscript have received financial support from the National Institutes of Health (T.L.S.B., J.C.M., B.M.A., C.X.), National Institute on Aging (J.J.L.G., A.J.A.), Alzheimer’s Association (J.J.L.G., P.R.M.), BrightFocus Foundation (P.R.M.), Avid Radiopharmaceuticals/Eli Lilly (T.L.S.B.), and Siemens (T.L.S.B.). Travel support was received from the NIA (P.R.M., A.J.A.), Longer Life Foundation for the AAIM meeting (J.C.M.), AD/PD meeting (J.C.M.), ATRI/ADNI Investigators meeting (J.C.M.), ADRC spring meeting (J.C.M.), DIAN symposium (J.C.M.), ADC meeting (J.C.M.), International Conference for Healthy Aging & Biomarkers (J.C.M.), and International Brain Health Symposium (J.C.M.). Consultations have been declared for Biogen (T.L.S.B.), Eli Lilly (T.L.S.B.), Eisai (T.L.S.B.), Bristol Myers Squibb (T.L.S.B.), Johnson & Johnson (T.L.S.B.), Barcelona Brain Research Center (J.C.M.), Native Alzheimer Disease-Related Resource Center in Minority Aging Research (J.C.M.), AlzPath (J.H.), Prothena (J.H.), Diadem (C.X.), and Albert Einstein College of Medicine (A.J.A.). Honoraria or payment was received from Medscape (T.L.S.B.), Peer View (T.L.S.B.), Longer Life Foundation for the AAIM meeting (J.C.M.), and International Brain Health Symposium (J.C.M.). Patents have been declared for Diffusion Basis Spectrum Imaging (DBSI), a novel diffusion MRI method used to quantify neuroinflammation and predict Alzheimer’s Disease progression (T.L.S.B.). Authors participated on advisory boards for Eisai (T.L.S.B.), Siemens (T.L.S.B.), Cure Alzheimer’s Fund Research Strategy Council (J.C.M.), Indiana University LEADS Advisory Board (J.C.M.), FDA Advisory Committee on Imaging Medical Products (C.X.), and NIH-sponsored external advisor grants (T.L.S.B.). Authors additionally held leadership or fiduciary roles for the ASNR Alzheimer’s and ARIA Study Group (T.L.S.B.), QIBA Amyloid PET Working Group (T.L.S.B.), Alzheimer’s Association Clinical Tau PET Work Group (T.L.S.B.), American College of Radiology/AlzNet Work Group (T.L.S.B.), RSNA QUIC (T.L.S.B.), and NIH CNN Study Section (T.L.S.B.). Precursors for radiopharmaceuticals and/or technology transfer were received from Avid Radiopharmaceuticals/Eli Lilly (T.L.S.B.), LMI (T.L.S.B.), Cerveau (T.L.S.B.), and Hyperfine (T.L.S.B.). Washington University School of Medicine in St. Louis has a financial interest in C2N Diagnostics and may financially benefit if the company is successful in marketing its product(s) that are related to this research. The current study is not directly concerned by this statement as it does not utilize data from this project. All other authors have nothing to disclose.
Figures




References
-
- Armitage , S. G. ( 1946. ). An analysis of certain psychological tests used for the evaluation of brain injury . Psychological Monographs , 60 ( 1 ), i – 48 . 10.1037/h0093567 - DOI
-
- Aschenbrenner , A. J. , Balota , D. A. , Fagan , A. M. , Duchek , J. M. , Benzinger , T. L. S. , & Morris , J. C. ( 2015. ). Alzheimer disease cerebrospinal fluid biomarkers moderate baseline differences and predict longitudinal change in attentional control and episodic memory composites in the adult children study . Journal of the International Neuropsychological Society , 21 ( 8 ), 573 – 583 . 10.1017/S1355617715000776 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
