Emerging gut microbial glycoside hydrolase inhibitors
- PMID: 40534733
- PMCID: PMC12171861
- DOI: 10.1039/d5cb00050e
Emerging gut microbial glycoside hydrolase inhibitors
Abstract
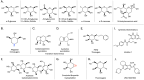
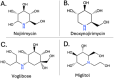
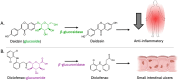
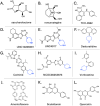
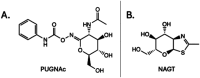
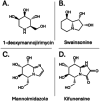
The human gut microbiota has been linked to numerous diseases through their metabolism of molecules in the gastrointestinal tract. Post-translational glycosylation is applied to many secreted proteins, including mucins and immunoglobulins, and glycosides are present in diet and generated by host metabolism systems. Thus, glycosides are key targets for degradation by gut microbial glycoside hydrolases (GHs). Indeed, diverse xenobiotic compounds, including therapeutics and dietary phytochemicals, along with endobiotics like neurotransmitters and hormones, are conjugated to monosaccharides making them substrates for GH enzymes. A range of GH inhibitors have been developed to study lysosomal storage diseases, treat viral infections, and to address type II diabetes. Recently, GH inhibitors have offered promising avenues for investigating gut microbial GHs and their influence on host health and disease. In this review we describe the growing classes of GH inhibitors and their applications in studying gut microbial GHs that target host-derived glycans and dietary and drug-xenobiotic molecules. We also review the use of GH-targeting activity-based probes to pinpoint specific proteins expressed by the gut microbiota that influence molecular and phenotypic outcomes. As we deepen our understanding of gut microbial GH function, we will further elucidate the roles played by the microbiota in host physiology and disease toward potential therapeutic interventions that target non-host factors in acute and chronic disorders.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
MRR is a founder of Symberix, Inc., and has received research funding from Lilly and Merck.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

