PPARα deficiency exacerbates retinal pathological changes and dysfunction in high-fat diet mice
- PMID: 40534810
- PMCID: PMC12120453
- DOI: 10.18240/ijo.2025.06.03
PPARα deficiency exacerbates retinal pathological changes and dysfunction in high-fat diet mice
Abstract
Aim: To examined the effects of a high-fat diet (HFD) on retinal pathological changes and dysfunction using peroxisome proliferator-activated receptor-alpha (PPARα) knockout mice.
Methods: For four months, C57BL/6J and PPARα knockout mice received either HFD or a standard diet (SD). A fluorometric method was used to determine the retinal triglycerides. The retinal malondialdehyde (MDA) content was measured. Hematoxylin-eosin was used to evaluate retinal pathological changes. Protein expression was analyzed by Western blot and immunofluorescence, while mRNA expression was evaluated by quantitative reverse transcription-polymerase chain reaction. Electroretinogram was used to assess retinal function.
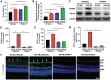
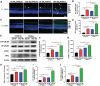
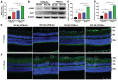
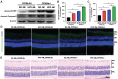
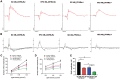
Results: HFD resulted in increased fatty acid β-oxidation in the inner retina, particularly retinal ganglion cells (RGCs), as well as increased weight and accumulation of retinal triglyceride. Retinal fatty acid β-oxidation and triglyceride accumulation were affected by PPARα -/- abnormalities. PPARα knockdown increased the infiltration and activation of inflammatory cells, as well as it upregulated the nuclear factor kappa B (NF-κB) signaling pathway and corresponding proinflammatory cytokine levels in the most retina subjected to the HFD. In the HFD mice, oxidative stress levels were elevated in the inner retina, particularly in the HFD PPARα -/- mice. HFD-induced RGCs apoptosis initiation was exacerbated by PPARα deficiency. Lastly, HFD feeding resulted in the lower amplitudes of scotopic a-wave, b-wave and photopic negative response (PhNR) wave, particularly in HFD PPARα -/- mice.
Conclusion: In HFD-fed mice retina, particularly in the inner retina, PPARα knockout increases lipid metabolic abnormalities, inflammatory responses, oxidative stress, apoptosis initiation and dysfunction.
Keywords: electroretinogram; high-fat diet; peroxisome proliferator-activated receptor-alpha; retina.
International Journal of Ophthalmology Press.
Conflict of interest statement
Conflicts of Interest: Wang X, None; Ding JJ, None; Yu CF, None; Xiao DC, None; Tao LM, None; Jiang ZX, None.
Figures
References
LinkOut - more resources
Full Text Sources
