Role of CD28+ PD-1+ Tc cells in immune response and prognosis prediction in hepatocellular carcinoma
- PMID: 40534863
- PMCID: PMC12174045
- DOI: 10.3389/fimmu.2025.1576193
Role of CD28+ PD-1+ Tc cells in immune response and prognosis prediction in hepatocellular carcinoma
Abstract
Background: CD28+PD-1+ Tc cells (CD8+ T cells) constitute a dysfunctional subset of T cell; however, the mechanisms underlying their dysfunction and their significance in hepatocellular carcinoma (HCC) remain unclear. We aimed to elucidate the prognostic significance and molecular characteristics of CD28+PD-1+ Tc cell infiltration in HCC.
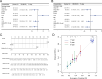
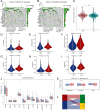
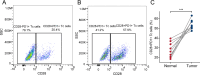
Methods: We established a single-cell HCC transcriptional map, focusing on cell-cell communication and trajectory analysis of CD28+PD-1+ Tc cells. We assessed the correlation between CD28+PD-1+ Tc-cell enrichment and prognosis and investigated potential molecular mechanisms using enrichment analyses. Flow cytometry was used to compare CD28+PD-1+ Tc-cell infiltration between HCC and adjacent normal tissues and cytotoxic factors and immune checkpoint expression were evaluated.
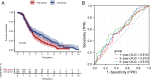
Results: Overall, 25,644 T cells were identified from single-cell RNA sequencing data from 10 HCC samples and corresponding normal samples. Overall T-cell infiltration was lower in HCC tissues, with significantly higher CD28+PD-1+ Tc-cell infiltration. Bulk RNA sequencing data integration revealed a correlation between higher CD28+PD-1+ Tc-cell infiltration and significantly worse prognosis. Flow cytometry confirmed higher CD28+PD-1+ Tc-cell enrichment in HCC tissues. Additionally, cytotoxic factor expression was significantly lower in CD28+PD-1+ Tc cells than in CD28-PD-1+ Tc cells, with lower expression of TIGIT and TIM-3 immune checkpoint molecules.
Conclusions: Significantly high CD28+PD-1+ Tc-cell enrichment in HCC indicates potential immune dysfunction. CD28+PD-1+ Tc-cell enrichment may serve as a sensitive prognostic marker and indicator for predicting treatment responses.
Keywords: CD28; PD-1; hepatocellular carcinoma; single-cell RNA-seq; t cell exhaustion; tumor microenvironment.
Copyright © 2025 Yang, Pan, Chen, Guo, Peng, Wang, Peng and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

