Computational design and evaluation of multiepitope vaccines against herpes simplex virus type 1
- PMID: 40534865
- PMCID: PMC12174051
- DOI: 10.3389/fimmu.2025.1581571
Computational design and evaluation of multiepitope vaccines against herpes simplex virus type 1
Abstract
Introduction: Herpes simplex virus type 1 (HSV-1) is a prevalent human pathogen, causing infections in various tissues and leading to severe complications such as herpes simplex encephalitis and cognitive impairments. Despite existing antiviral treatments, recurrent infections and the lack of effective vaccines highlight the need for new preventive measures.
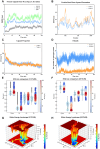
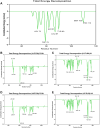
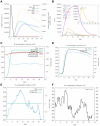
Methods: We employed immunogenomic and bioinformatics methods to design two multi-epitope vaccine constructs 1 and 2 against HSV-1. The Immune Epitope Database was used to identify B-cell and T-cell epitopes from HSV-1 glycoproteins. The IFN epitope server and the IL4pred/IL-10pred server were used to ascertain the activation possibility of IFN-γ, IL-4, and IL-10. The NetMHC-4.0 and NetMHCII2.3 servers were used to identify MHC epitopes. The constructed vaccine was analyzed for antigenicity and allergenicity using the VaxiJen v2.0 and AllergenFP servers. The three-dimensional structure of the vaccine construct was constructed using the AlphaFold3 tool. The ClusPro 2.0 server was utilized for molecular docking and the Desmond module in Schrodinger 2021-1 was utilized for molecular dynamics and MM/PBSA analysis. The immunogenicity and the corresponding immune response curves were analyzed using the C-ImmSim server.
Results: Bioinformatics analysis demonstrated that these vaccines exhibited both good affinity and immunogenicity, and were non-toxic and non-allergenic to the host. In addition, vaccine construct 2 exhibits superior stability and binding affinity with TLR9, and is more effective in triggering a robust immune response.
Discussion: This approach targets the key mechanisms of HSV-1 entry and TLR-mediated immune responses, providing a potential strategy for preventing and treating HSV-1 infections. Furthermore, the identified and optimized vaccine construct offers a promising avenue for developing a preventive vaccine against HSV-1, addressing the critical need for better control of this widespread virus.
Keywords: HSV-1; immune response; molecular docking; molecular dynamics simulation; multiepitope vaccine.
Copyright © 2025 Zhao, Wang, Pang, Zhou, Li, Wang, Zheng and Ren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

