Tumor marker pseudoprogression and immune-related cholangitis during conversion therapy for massive hepatocellular carcinoma: a case report
- PMID: 40534866
- PMCID: PMC12174441
- DOI: 10.3389/fimmu.2025.1529016
Tumor marker pseudoprogression and immune-related cholangitis during conversion therapy for massive hepatocellular carcinoma: a case report
Abstract
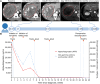
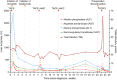
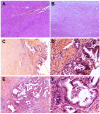
Cases with massive (diameter ≥10 cm) hepatocellular carcinomas (HCCs) are uncommon and typically have poor outcomes; however, conversion therapy offers a beacon of hope for remission in patients with massive unresectable HCCs. Recently, immune checkpoint inhibitors (ICIs) have been used in combination with other treatment modalities to improve the response rates to conversion therapies, yet the safety and generalizability of this combination have not been extensively validated. Herein, we report a man with a chief complaint of abdominal pain who was diagnosed with massive unresectable HCC. Notably, the patient successfully underwent curative surgery after quadruple conversion therapy using tislelizumab (an ICI), lenvatinib, transarterial chemoembolization, and hepatic arterial infusion chemotherapy directed by a multidisciplinary team. With a complete response achieved, this case demonstrated the major potential of this combination regimen for HCC, and the remarkable efficacy was also reflected by substantial reductions in both alpha-fetoprotein and des-gamma-carboxy prothrombin overall. Nevertheless, transient increases in both biomarkers (tumor marker pseudoprogression) were observed within the first three weeks after initiating ICI treatment. Furthermore, the patient developed a biliary stricture, which resolved after discontinuing the ICI and was ultimately assessed as an immune-related adverse event. Therefore, in the context of combination therapy, further evaluation of the robustness of tumor markers is warranted, and it is crucial for clinicians to be mindful of potential immune-related adverse events.
Keywords: case report; conversion therapy; hepatocellular carcinoma; immune checkpoint inhibitor; immune-related adverse event; pseudoprogression; tislelizumab; tumor marker.
Copyright © 2025 Zhang, Zhang, Zhao, Dai, Li, Wu, Yan, Lin and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Sintilimab plus lenvatinib in combination with transarterial chemoembolization and subsequent radiofrequency ablation for unresectable hepatocellular carcinoma: a single-arm, single-center study.Sci Rep. 2025 Jul 25;15(1):27123. doi: 10.1038/s41598-025-12858-y. Sci Rep. 2025. PMID: 40715509 Free PMC article.
-
Conversion study of hepatocellular carcinoma using HAIC combined with lenvatinib and PD-1/L1 immunotherapy under the guidance of BCLC staging.Front Immunol. 2025 Jun 2;16:1596864. doi: 10.3389/fimmu.2025.1596864. eCollection 2025. Front Immunol. 2025. PMID: 40529364 Free PMC article.
-
Case Report: Durable complete response of advanced-stage hepatocellular carcinoma to DEB-TACE combined with lenvatinib and camrelizumab.Front Immunol. 2025 Jun 20;16:1549675. doi: 10.3389/fimmu.2025.1549675. eCollection 2025. Front Immunol. 2025. PMID: 40621454 Free PMC article.
-
Surgical resection of vascular-invasive late-stage hepatocellular carcinoma following transarterial chemoembolization combined with lenvatinib and tislelizumab: Two case reports and literature review.Medicine (Baltimore). 2025 Jun 20;104(25):e42973. doi: 10.1097/MD.0000000000042973. Medicine (Baltimore). 2025. PMID: 40550061 Free PMC article. Review.
-
Clinical efficacy of lenvatinib, trans-arterial chemoembolization, and PD-1/L1 inhibitors in advanced hepatocellular carcinoma: a systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2652-2664. doi: 10.1007/s12094-024-03458-9. Epub 2024 Apr 26. Clin Transl Oncol. 2024. PMID: 38671328
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

