Temporal changes in the protein cargo of extracellular vesicles and resultant immune reprogramming after severe burn injury in humans and mice
- PMID: 40534873
- PMCID: PMC12173884
- DOI: 10.3389/fimmu.2025.1596598
Temporal changes in the protein cargo of extracellular vesicles and resultant immune reprogramming after severe burn injury in humans and mice
Abstract
Introduction: Severe injury, including burn trauma, leads to profound immune dysfunction, yet the mechanisms driving these changes remain incompletely defined. This lack of understanding has hindered efforts to modulate the immune response effectively. Additionally, a clear biomarker profile to guide clinicians in identifying burn patients at high risk for poor clinical outcomes is lacking. Extracellular vesicles (EVs) have emerged as novel mediators of immune dysfunction in various pathologies. Prior studies in mouse models have demonstrated that plasma EVs increase following burn injury and contribute to immune dysfunction. Furthermore, EVs have potential as biomarkers for predicting extended hospital stays in burn patients. This study hypothesizes that human EVs, purified early and late after burn injury, will exhibit immune reprogramming effects similar to those observed in mice and that specific EV protein cargo may serve as biomarkers of immune and physiological responses to burn injury.
Methods: EVs were isolated from the plasma of burn-injury patients at early (<72h) and late (≥14 days) time points post-injury. Using unbiased immune transcriptome and bioinformatic causal network analyses, the immunomodulatory effects of these EVs were assessed in human THP-1 macrophages. Mass spectrometry-based quantitative proteomics and pathway analyses were conducted to characterize the protein cargo of EVs from both human and mouse models at different post-burn phases.
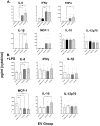
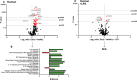
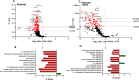
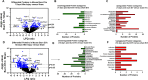
Results: Early post-burn human EVs induced significant immune reprogramming in macrophages, increasing pro-inflammatory signaling while suppressing anti-inflammatory pathways. In contrast, late post-burn EVs exhibited an immunosuppressive profile, with downregulation of pro-inflammatory pathways and upregulation of anti-inflammatory signaling. Proteomic analyses revealed that human and mouse EVs contained unique and overlapping protein cargo across different time points. At day 7 post-burn, mouse EVs were enriched in circulation/complement and neuronal proteins, whereas by day 14, reductions in membrane and metabolism-associated proteins were observed. Similarly, in human EVs at 14 days post-burn, increased levels of circulation/complement, immune, and transport proteins were detected.
Conclusions: EVs from burn-injury patients at distinct time points differentially modulate immune responses in macrophages, mirroring the temporal immune phenotypes observed in clinical settings. These findings suggest that EV-macrophage interactions play a crucial role in burn-induced immune dysfunction and highlight the potential of EV protein cargo as biomarkers for immune status and patient outcomes following burn injury.
Keywords: burn injury; exosomes; extracellular vesicles; macrophages; microvesicles; thermal injury.
Copyright © 2025 Willis, Seim, Herring, Mordant, Webb, Upchurch, Sharma, Cairns, Efron, Wallet, Coleman and Maile.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Update of
-
Temporal changes in the protein cargo of extracellular vesicles and resultant immune reprogramming after severe burn injury in humans and mice.bioRxiv [Preprint]. 2025 Mar 19:2025.03.19.644202. doi: 10.1101/2025.03.19.644202. bioRxiv. 2025. Update in: Front Immunol. 2025 Jun 04;16:1596598. doi: 10.3389/fimmu.2025.1596598. PMID: 40166336 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

