Partial depletion of circulating neutrophil granulocytes in mice exacerbates the inflammatory response and hypothermia during LPS induced severe systemic inflammation
- PMID: 40534875
- PMCID: PMC12173888
- DOI: 10.3389/fimmu.2025.1578590
Partial depletion of circulating neutrophil granulocytes in mice exacerbates the inflammatory response and hypothermia during LPS induced severe systemic inflammation
Abstract
Introduction: During acute inflammation, immune-to-brain signaling plays a pivotal role in the generation of sickness responses such as fever or hypothermia. Neutrophil granulocytes (NG) are a crucial component of the immune system and modulate inflammation. Moreover, neutropenic fever is a severe condition for immunocompromised patients that can be life threatening. Using a mouse model of partial NG depletion, we aimed to investigate how neutropenia alters immune-to-brain signaling and the development of sickness responses during high-dose-LPS-induced inflammation.
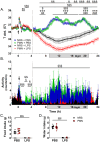
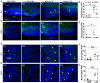
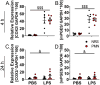
Methods: To deplete NGs, mice were injected intraperitoneally (IP) with heterologous anti-polymorphonuclear leukocyte serum at 1:4 ratio in PBS (PMN, 1.82 mg/kg IgG) or normal rabbit serum (NRS, 1 mg/kg IgG) as a control. To induce inflammation, mice were injected IP with lipopolysaccharide (LPS, 2.5 mg/kg) or PBS as a control 24 h after PMN or NRS. Physiological parameters were documented using a telemetric system that continuously recorded: food and water intake, locomotor activity, and core body temperature. At 4 h or 24 h after LPS-stimulation, brain and serum samples were collected and analyzed for peripheral and brain inflammatory markers.
Results: After stimulation with LPS, PMN-pretreated mice showed neutropenia (significantly by ~25% of the control value) and attenuated NG recruitment to the brain in a structure dependent manner. LPS-induced hypothermia was more severe in PMN-pretreated mice while other physiological parameters were only altered by LPS alone. Additional analyses in NG depleted mice revealed that corticosterone levels showed an early reduced but late increased magnitude, and circulating cytokines like interleukin-10 were exacerbated during LPS-induced inflammation. Despite a weak overall impact on the brain, the hypothalamus of neutropenic mice presented exacerbated LPS-induced levels of IL-6, a key mediator of inflammation, compared to immunocompetent control mice.
Discussion: Overall, we found that partial NG depletion exaggerates the peripheral inflammatory response and this strong peripheral reaction may contribute to the exacerbation of sickness symptoms most likely involving circulating IL-10 with strong implications for clinical cases of neutropenic patients.
Keywords: hypothermia; immune-to-brain signaling; inflammation; neutropenia; neutrophil granulocytes; sickness response.
Copyright © 2025 Hernandez, Pflieger, Schäffer, Bähr, Schneiders, Reichel, Meurer, Lamp, Bauer, Krüger, Harden, von Köckritz-Blickwede and Rummel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Combined effects of agomelatine and 2600 MHz radiofrequency field on inflammation induced by chronic lipopolysaccharide administration.Electromagn Biol Med. 2025 Aug 12:1-10. doi: 10.1080/15368378.2025.2545871. Online ahead of print. Electromagn Biol Med. 2025. PMID: 40792433
-
Alleviation of lipopolysaccharide-induced heart inflammation in poultry treated with carnosic acid via the NF-κB and MAPK pathways.J Anim Sci. 2025 Jan 4;103:skae373. doi: 10.1093/jas/skae373. J Anim Sci. 2025. PMID: 39657120
-
PMN-MDSCs are responsible for immune suppression in anti-PD-1 treated TAP1 defective melanoma.Clin Transl Oncol. 2025 Jul;27(7):3073-3083. doi: 10.1007/s12094-024-03840-7. Epub 2025 Jan 18. Clin Transl Oncol. 2025. PMID: 39825997
-
Systemic inflammation and microglial activation: systematic review of animal experiments.J Neuroinflammation. 2015 Jun 6;12:114. doi: 10.1186/s12974-015-0332-6. J Neuroinflammation. 2015. PMID: 26048578 Free PMC article.
-
Defining disease severity in atopic dermatitis and psoriasis for the application to biomarker research: an interdisciplinary perspective.Br J Dermatol. 2024 Jun 20;191(1):14-23. doi: 10.1093/bjd/ljae080. Br J Dermatol. 2024. PMID: 38419411 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

