Dosing Recommendations for Ampicillin and Ceftriaxone in the Treatment of Pediatric Community-Acquired Pneumonia Using Monte Carlo- and Physiologic-Based Pharmacokinetic Simulations
- PMID: 40534929
- PMCID: PMC12172679
- DOI: 10.5863/JPPT-24-00069
Dosing Recommendations for Ampicillin and Ceftriaxone in the Treatment of Pediatric Community-Acquired Pneumonia Using Monte Carlo- and Physiologic-Based Pharmacokinetic Simulations
Abstract
Objective: Since 2011, Ampicillin (AMP) has been recommended as the parenteral antibiotic of choice for pediatric community-acquired pneumonia (CAP), but ceftriaxone (CRO) is recommended for unvaccinated children and those with complicated CAP. Using penicillin and CRO susceptibility data for pneumococcus, we evaluated the adequacy of currently recommended doses of AMP and CRO.
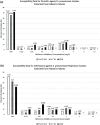
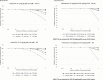
Methods: With nonlinear mixed-effects modeling v7.3, Monte Carlo simulations (MCS, N = 10,000) for AMP and CRO were conducted for 6 virtual patients aged 3 months, 1, 2, 5, 10, and 15 years. PK-Sim v9.0 was used to develop physiologic-based pharmacokinetic (PBPK) models for AMP (N = 4000) and CRO (N = 3000). The probability of target attainment (PTA) was determined for both serum and lung (epithelial lining fluid [ELF]) exposure to achieve free drug concentrations above the minimum inhibitory concentration (%fT>MIC) for pneumococci at 30% to 50% of the dosing interval.
Results: We performed simulations based on susceptibility data from 21 pneumococci isolated from children with CAP and found all 21 (100%) to be susceptible to AMP and CRO using Clinical & Laboratory Standard Institute/US Food and Drug Administration breakpoints, where susceptible, intermediate, and resistant strains of Streptococcus pneumoniae were ≤1, 2, and ≥4 mg/L for CRO and ≤2, 4, and ≥8 mg/L for AMP (extrapolated from penicillin), respectively (where intermediate and resistant were considered nonsusceptible); and 18 (85.7%) were susceptible to AMP, and 19 (90.5%) to CRO using the European Committee on Antimicrobial Susceptibility Testing/European Medicines Agency breakpoints, where susceptible and nonsusceptible strains were as follows: 0.5 and 2 mg/L for CRO and 0.5 and 1 mg/L for AMP. Both the serum and ELF, antibiotic regimens achieved >99% PTA at 30% to 50% fT>MIC using MCS and PBPK.
Conclusion: In the pneumococcal conjugate era, standard doses of AMP and CRO appear to provide the appropriate serum and ELF exposure for clinical and microbiologic success for >98% of children with pediatric CAP. The required dose to achieve the desired outcomes may change if beta-lactam resistance in pneumococcus increases.
Keywords: Monte Carlo simulation; Streptococcus pneumoniae; beta-lactams; children; pharmacodynamic; physiologic-based pharmacokinetic simulation; pneumococcal vaccine.
Copyright. Pediatric Pharmacy Association. All rights reserved. For permissions, email: membership@pediatricpharmacy.org.
Figures


Similar articles
-
Amoxicillin and penicillin G dosing in pediatric community-acquired pneumococcal pneumonia in the era of conjugate pneumococcal vaccines.Pharmacotherapy. 2024 Aug;44(8):606-614. doi: 10.1002/phar.2756. Epub 2023 Jan 9. Pharmacotherapy. 2024. PMID: 36571459
-
Re-evaluation of penicillin and ceftriaxone MIC results to predict susceptibility to the oral cephalosporin, cefpodoxime, in Streptococcus pneumoniae clinical isolates from the United States according to CLSI guidelines (2019-2021).J Clin Microbiol. 2025 Aug 13;63(8):e0002725. doi: 10.1128/jcm.00027-25. Epub 2025 Jun 24. J Clin Microbiol. 2025. PMID: 40552822 Free PMC article.
-
Current Ceftriaxone Dose Recommendations are Adequate for Most Critically Ill Children: Results of a Population Pharmacokinetic Modeling and Simulation Study.Clin Pharmacokinet. 2021 Oct;60(10):1361-1372. doi: 10.1007/s40262-021-01035-9. Epub 2021 May 26. Clin Pharmacokinet. 2021. PMID: 34036552 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy.Cochrane Database Syst Rev. 2019 Nov 20;2019(11):CD012024. doi: 10.1002/14651858.CD012024.pub3. Cochrane Database Syst Rev. 2019. PMID: 31745984 Free PMC article.
References
-
- Bradley JS, Byington CL, Shah SS et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76. - PMC - PubMed
-
- Turnidge JD. The pharmacodynamics of beta-lactams. Clin Infect Dis. 1998;27(1):10–22. - PubMed
-
- Kuti J. Optimizing antimicrobial pharmacodynamics: a guide for your stewardship program. Rev Med Clin Condes. 2016;5(27):615–624.
-
- Levy C, Biscardi S, Dommergues MA et al. Impact of PCV13 on community-acquired pneumonia by C-reactive protein and procalcitonin levels in children. Vaccine. 2017;35(37):5058–5064. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
