Evolution of Thermal Plasticity in Hymenoscyphus fraxineus During Ash Dieback Expansion in Europe
- PMID: 40534982
- PMCID: PMC12173838
- DOI: 10.1002/ece3.71513
Evolution of Thermal Plasticity in Hymenoscyphus fraxineus During Ash Dieback Expansion in Europe
Abstract
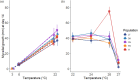
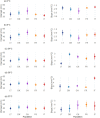
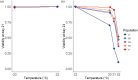
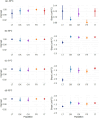
The plasticity of adaptive traits may be critical for population persistence in heterogeneous environments. However, its evolution is rarely investigated in forest pathogens, potentially limiting the accuracy of epidemic risk predictions. Ash dieback is an emblematic example of a forest epidemic caused by an invasive fungal pathogen-Hymenoscyphus fraxineus, which has likely been introduced to Eastern Europe from East Asia. We investigated the plasticity and thermal niche evolution of H. fraxineus during its spread across Europe. We characterized the reaction norms of in vitro mycelial growth and viability of H. fraxineus isolates from five European populations sampled along a latitudinal gradient spanning from Lithuania to Italy. While all populations responded uniformly to temperature decrease, their responses to temperature increase diverged markedly. The growth of H. fraxineus isolates from the northernmost population (Lithuania) was most negatively affected by high temperatures, whereas the southernmost isolates (Italy) showed optimal growth at a higher temperature compared to the other populations. Additionally, the viability of Lithuanian isolates was significantly reduced by higher temperatures compared to that of the other populations. These findings suggest that both growth plasticity and thermal niche have evolved during the pathogen's expansion in Europe, with potentially important implications for predicting and managing future epidemic risks. We further discuss how evolutionary processes may have shaped these phenotypic differences.
Keywords: Chalara fraxinea; Hymenoscyphus fraxineus; Hymenoscyphus pseudoalbidus; ash dieback; invasive fungal pathogen; microbial evolution; plasticity evolution; thermal plasticity.
© 2025 The Author(s). Ecology and Evolution published by British Ecological Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Adamčíková, K. , Pažitný J., and Pastirčáková K.. 2018. “Individual Resistance of Fraxinus angustifolia and F. excelsior Clones to Hymenoscyphus fraxineus .” Journal of Plant Protection Research 58: 227–233. 10.24425/122937. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

