Updated long-term survival outcomes for patients with locally advanced gastric cancer having pathological complete response after neoadjuvant therapy at China National Cancer Center, 2004-2023
- PMID: 40535119
- PMCID: PMC12173866
- DOI: 10.3389/fonc.2025.1539534
Updated long-term survival outcomes for patients with locally advanced gastric cancer having pathological complete response after neoadjuvant therapy at China National Cancer Center, 2004-2023
Abstract
Background: Neoadjuvant chemotherapy increases the probability of achieving negative margins and may even lead to pathological complete response (pCR) in locally advanced gastric cancer (LAGC). The incorporation of neoadjuvant immunotherapy is promising in further enhancing the pCR rate. However, long-term survival outcomes and factors affecting the prognosis of pCR patients have not been fully elucidated.
Patients and methods: We conducted a retrospective analysis of all patients who achieved pCR between January 2004 and June 2023. Cox regression models were used to identify clinicopathological predictors of overall survival (OS) and disease-free survival (DFS). Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test.
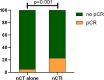
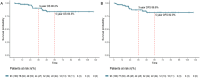
Results: After screening, 112 patients were included in the study, with a median follow-up time of 42 (range: 2-117) months and a pCR rate of 7.4%. The 3- and 5-year OS rates were 90.2% and 83.3%, respectively, while the 3- and 5-year DFS rates were 86.8% and 82.0%, respectively. Within the multivariate Cox model, neoadjuvant chemotherapy was a prognostic factor for improved OS and DFS. There was no statistically significant disparity in OS and DFS between patients who received postoperative adjuvant therapy and those who did not. Moreover, the combination of neoadjuvant immunotherapy with chemotherapy, as compared to neoadjuvant chemotherapy alone, substantially increased the pCR rate (p <0.001).
Conclusions: Patients with LAGC who achieved pCR demonstrated favorable long-term survival outcomes, with no additional survival benefits conferred by adjuvant therapy. Although neoadjuvant immunotherapy increased the pCR rate, its impact on the prognosis of pCR patients requires further investigation.
Keywords: gastric cancer; immunotherapy; neoadjuvant therapy; pathological complete response (pCR); prognostic factor.
Copyright © 2025 Sun, Wang, Zhang, Zhao, Niu, Wang, Luan, Han, Chen and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
[Analysis of the safety and efficacy of neoadjuvant immunotherapy combined with chemotherapy for radical resection of locally advanced gastric cancer: a two-center propensity-matched study].Zhonghua Wai Ke Za Zhi. 2025 Aug 20;63(10):953-962. doi: 10.3760/cma.j.cn112139-20250422-00214. Online ahead of print. Zhonghua Wai Ke Za Zhi. 2025. PMID: 40831135 Chinese.
-
Enhancing survival in locally advanced esophageal cancer: a comparative analysis of neoadjuvant immunotherapy versus conventional neoadjuvant therapies using the SEER database.J Thorac Dis. 2025 May 30;17(5):2778-2801. doi: 10.21037/jtd-24-1905. Epub 2025 May 28. J Thorac Dis. 2025. PMID: 40529733 Free PMC article.
-
Development and validation of a Log odds of negative lymph nodes/T stage ratio-based prognostic model for gastric cancer.Front Oncol. 2025 Jun 3;15:1554270. doi: 10.3389/fonc.2025.1554270. eCollection 2025. Front Oncol. 2025. PMID: 40530015 Free PMC article.
-
Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy.Cochrane Database Syst Rev. 2019 Nov 20;2019(11):CD012024. doi: 10.1002/14651858.CD012024.pub3. Cochrane Database Syst Rev. 2019. PMID: 31745984 Free PMC article.
-
Prevalence and odds of anxiety and depression in cutaneous malignant melanoma: a proportional meta-analysis and regression.Br J Dermatol. 2024 Jun 20;191(1):24-35. doi: 10.1093/bjd/ljae011. Br J Dermatol. 2024. PMID: 38197404
References
-
- Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. (2016) 17:1697–708. doi: 10.1016/S1470-2045(16)30531-9 - DOI - PubMed
-
- Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. (2021) 22:1081–92. doi: 10.1016/S1470-2045(21)00297-7 - DOI - PubMed
LinkOut - more resources
Full Text Sources

