Efficacy and safety of PI3K inhibitors combined with fulvestrant for HR+/HER2- advanced breast cancer: a systematic review and meta-analysis
- PMID: 40535135
- PMCID: PMC12174159
- DOI: 10.3389/fonc.2025.1556978
Efficacy and safety of PI3K inhibitors combined with fulvestrant for HR+/HER2- advanced breast cancer: a systematic review and meta-analysis
Abstract
Objectives: This systematic review and meta-analysis aimed to evaluate the efficacy and safety of combination of Phosphatidylinositol 3-kinase (PI3K) inhibitors and fulvestrant in patients with advanced breast cancer (ABC) who are hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-).
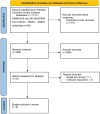
Methods: A systematic search was conducted across the PubMed, Cochrane Library, EMBASE databases and major conference websites (ASCO, ESMO, SABCS) to identify randomized controlled trials (RCTs) evaluating the combination of PI3K inhibitors and fulvestrant in the treatment of advanced breast cancer. Two independent reviewers systematically screened the literature, extracted data, and assessed the risk of bias for the included studies based on predefined criteria. Meta-analysis was subsequently performed using R software in accordance with the PRISMA guidelines.
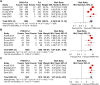
Results: A total of five randomized controlled trials (RCTs) involving 3,011 patients were included. The findings indicated that the combination of PI3K inhibitors and fulvestrant significantly improved progression-free survival (PFS) (HR = 0.74, 95% CI 0.67-0.80, P < 0.0001) and the objective response rate (ORR) (RR = 1.80, 95% CI: 1.39-2.35, P < 0.0001) compared to placebo plus fulvestrant. However, there was no statistically significant difference in clinical benefit rate (CBR) (RR = 1.10, 95% CI: 0.97-1.25, P = 0.1341). Subgroup analysis indicated that the predefined subgroup of PFS based on PIK3CA mutation status assessed by ctDNA was statistically significant (P = 0.0039), whereas the predefined subgroup of PFS based on PIK3CA mutation status assessed by tumor tissue was not statistically significant (P = 0.1514). In terms of adverse events, the incidence of grade ≥3 events was significantly increased in the PI3K inhibitors combined with fulvestrant group (RR=2.11, 95% CI: 1.73-2.58, P<0.0001), particularly hyperglycemia, rash, and transaminitis (ALT).
Conclusion: The combination of PI3K inhibitors and fulvestrant significantly improved PFS and ORR in patients with advanced breast cancer. However, substantial dose-limiting toxicities associated with this therapeutic regimen restrict its broader clinical application. In patients with PIK3CA mutations detected on ctDNA analysis, PFS was significantly improved compared to those with wild-type PIK3CA, suggesting that ctDNA-based PIK3CA mutation status may serve as a potential biomarker for treatment response.
Systematic review registration: PROSPERO, identifier CRD42023407466.
Keywords: PI3K inhibitors; PIK3CA mutations; advanced breast cancer; meta-analysis; progression-free survival.
Copyright © 2025 Li, Wang, Lin and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










Similar articles
-
Which Is the Most Appropriate PI3K Inhibitor for Breast Cancer Patients with or without PIK3CA Status Mutant? A Systematic Review and Network Meta-Analysis.Biomed Res Int. 2020 Dec 3;2020:7451576. doi: 10.1155/2020/7451576. eCollection 2020. Biomed Res Int. 2020. PMID: 33376736 Free PMC article.
-
Abemaciclib Plus Fulvestrant in Advanced Breast Cancer After Progression on CDK4/6 Inhibition: Results From the Phase III postMONARCH Trial.J Clin Oncol. 2025 Mar 20;43(9):1101-1112. doi: 10.1200/JCO-24-02086. Epub 2024 Dec 18. J Clin Oncol. 2025. PMID: 39693591 Free PMC article. Clinical Trial.
-
Tibremciclib or Placebo Plus Fulvestrant in Hormone Receptor-Positive and ERBB2-Negative Advanced Breast Cancer After Endocrine Therapy: A Randomized Clinical Trial.JAMA Oncol. 2025 Jul 31:e252092. doi: 10.1001/jamaoncol.2025.2092. Online ahead of print. JAMA Oncol. 2025. PMID: 40742733 Free PMC article.
-
Efficacy and safety of entinostat plus exemestane in hormone receptor-positive breast cancer: a systematic review meta-analysis of randomized controlled trials.Breast Cancer Res Treat. 2025 Aug;212(3):417-426. doi: 10.1007/s10549-025-07737-z. Epub 2025 May 31. Breast Cancer Res Treat. 2025. PMID: 40448813 Review.
-
Efficacy and safety of combining re-irradiation with bevacizumab compared to bevacizumab alone in the management of recurrent high-grade gliomas: a meta-analysis and systematic review.Ther Adv Neurol Disord. 2025 Jun 14;18:17562864251343574. doi: 10.1177/17562864251343574. eCollection 2025. Ther Adv Neurol Disord. 2025. PMID: 40529988 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

