Exploiting induced pluripotent stem cell-derived retinal pigment epithelium to unravel host-pathogen interaction in ocular tuberculosis: a reverse translational in vitro model
- PMID: 40535229
- PMCID: PMC12173865
- DOI: 10.3389/fopht.2025.1610215
Exploiting induced pluripotent stem cell-derived retinal pigment epithelium to unravel host-pathogen interaction in ocular tuberculosis: a reverse translational in vitro model
Abstract
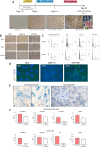
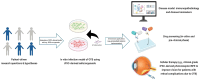
Mycobacterium tuberculosis (Mtb) can infect the retinal pigment epithelium (RPE) cells. Current in vitro research models for ocular tuberculosis (OTB) only rely on RPE cell culture approaches. Until now it remains unclear why only a minority of patients with active systemic tuberculosis (TB) develops concurrent OTB. There is significant variation in the clinical manifestations of OTB, which is potentially influenced by ethnic differences and diversity in mycobacterial strains. To better understand the immunopathobiology of OTB, particularly an individual's susceptibility to Mtb-infection and the specific host response, cell culture systems utilizing induced pluripotent stem cells (iPSC)-derived RPE cells offer a promising in vitro model to better mimic the disease. With this technology, RPE cells can be generated from specific patients of interest, enabling to test hypotheses in a bench to bedside or reverse manner. In this current study, we explore the utility of iPSC-derived RPE cells as an in vitro model for OTB. Such an approach would overcome drawbacks associated with the currently commonly used "general" RPE cell lines as disease model. The application of iPSC-derived RPE cells offers promising options for the identification of novel biomarkers and to study individualized drug screening methods for host-directed therapy of OTB, in order to restore and maintain vision in OTB patients with sight-threatening disease.
Keywords: induced pluripotent stem cells; personalized medicine; retinal pigment epithelium; tuberculosis; uveitis.
Copyright © 2025 Putera, de Meerendonk, Nagtzaam, La Distia Nora, Rombach, de Steenwinkel, Vingerling, Dik and van Hagen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Detection of Residual iPSCs Following Differentiation of iPSC-Derived Retinal Pigment Epithelial Cells.J Ocul Pharmacol Ther. 2024 Dec;40(10):680-687. doi: 10.1089/jop.2024.0130. Epub 2024 Oct 2. J Ocul Pharmacol Ther. 2024. PMID: 39358867
-
Current concepts in the diagnosis of ocular tuberculosis: A narrative review.Taiwan J Ophthalmol. 2025 Jun 10;15(2):203-211. doi: 10.4103/tjo.TJO-D-24-00115. eCollection 2025 Apr-Jun. Taiwan J Ophthalmol. 2025. PMID: 40584192 Free PMC article. Review.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Evaluation of Mycobacterium Tuberculosis Derived Cell-Free DNA-Based Multi-Targeted Real-Time PCR from Vitreous Fluid (VF) Samples to Diagnose Ocular Tuberculosis.Ocul Immunol Inflamm. 2025 Jul 21:1-6. doi: 10.1080/09273948.2025.2532820. Online ahead of print. Ocul Immunol Inflamm. 2025. PMID: 40689729
-
LipidUNet-Machine Learning-Based Method of Characterization and Quantification of Lipid Deposits Using iPSC-Derived Retinal Pigment Epithelium.J Vis Exp. 2023 Jul 28;(197):10.3791/65503. doi: 10.3791/65503. J Vis Exp. 2023. PMID: 37578220 Free PMC article.
References
-
- World Health Organization (WHO) . Global tuberculosis report 2024. Geneva: World Health Organization; (2024). Report No.: 9240101535.
-
- Ohara H, Harada Y, Hiyama T, Yamane K, Higaki M, Kobayashi T, et al. Incidence of ocular inflammation among patients with active tuberculosis or nontuberculous mycobacterial infections in a tertiary hospital in Japan. Int Ophthalmol. (2021) 41(4):1427–36. doi: 10.1007/s10792-021-01718-z - DOI - PubMed
LinkOut - more resources
Full Text Sources

