The Role of Pattern Recognition Receptors in Epigenetic and Metabolic Reprogramming: Insights into Trained Immunity
- PMID: 40535352
- PMCID: PMC12174933
- DOI: 10.2147/JIR.S513325
The Role of Pattern Recognition Receptors in Epigenetic and Metabolic Reprogramming: Insights into Trained Immunity
Abstract
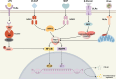
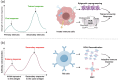
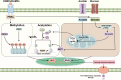
Pattern recognition receptors (PRRs) function as pivotal components of the innate immune system by orchestrating trained immunity through dynamic epigenetic and metabolic reprogramming. Recent discoveries demonstrate that PRRs not only detect pathogens but also actively regulate immune cell metabolism and transcriptional landscapes, thereby potentiating the speed and magnitude of defensive responses upon secondary challenges. These functional adaptations are coordinated through evolutionarily conserved signaling cascades that establish persistent immunological modifications at cellular and systemic levels. Nevertheless, despite substantial advances in characterizing PRR-driven immune activation, the molecular mechanisms governing their role in innate immune memory formation remain incompletely elucidated. This review systematically explores emerging paradigms of PRR-mediated epigenetic remodeling and metabolic rewiring, with particular emphasis on their mechanistic integration into trained immunity. We critically assess current evidence, identify unresolved questions regarding signal transduction specificity and memory maintenance, and propose novel methodological approaches to decipher the multilayered regulatory networks of innate immune adaptation. By elucidating these processes, our analysis establishes a conceptual framework for developing immunomodulatory therapies and leveraging trained immunity in precision medicine applications.
Keywords: epigenetic; immunocyte; metabolic reprogramming; pattern recognition receptors; trained immunity.
© 2025 Li et al.
Conflict of interest statement
The authors declare that this research was conducted without any commercial or financial relationships that could be perceived as potential conflicts of interest.
Figures
Similar articles
-
Drosophila melanogaster Toll-9 elicits antiviral immunity against Drosophila C virus.J Virol. 2025 Jun 17;99(6):e0221424. doi: 10.1128/jvi.02214-24. Epub 2025 May 14. J Virol. 2025. PMID: 40366172 Free PMC article.
-
Metabolic Regulation in the Induction of Trained Immunity.Semin Immunopathol. 2024 Jul 25;46(3-4):7. doi: 10.1007/s00281-024-01015-8. Semin Immunopathol. 2024. PMID: 39060761 Free PMC article. Review.
-
Pattern recognition receptors: function, regulation and therapeutic potential.Signal Transduct Target Ther. 2025 Jul 11;10(1):216. doi: 10.1038/s41392-025-02264-1. Signal Transduct Target Ther. 2025. PMID: 40640149 Free PMC article. Review.
-
Pattern recognition receptors and inflammasome: Now and beyond.Mol Cells. 2025 Aug;48(8):100239. doi: 10.1016/j.mocell.2025.100239. Epub 2025 Jun 15. Mol Cells. 2025. PMID: 40527410 Free PMC article. Review.
-
Pattern-Recognition Receptors and Immunometabolic Reprogramming: What We Know and What to Explore.J Innate Immun. 2024;16(1):295-323. doi: 10.1159/000539278. Epub 2024 May 13. J Innate Immun. 2024. PMID: 38740018 Free PMC article. Review.
Cited by
-
Macrophages in chronic infections: regulation and remodeling.Front Immunol. 2025 Jul 17;16:1594988. doi: 10.3389/fimmu.2025.1594988. eCollection 2025. Front Immunol. 2025. PMID: 40746542 Free PMC article. Review.
References
-
- Wicherska-Pawłowska K, Wróbel T, Rybka J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in innate immunity. TLRs, NLRs, and RLRs ligands as immunotherapeutic agents for hematopoietic diseases. Int J Mol Sci. 2021;22(24):13397. doi: 10.3390/ijms222413397 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

