Diagnostic value of ultrasonography for post-liver transplant hepatic vein complications
- PMID: 40535483
- PMCID: PMC11886290
- DOI: 10.5500/wjt.v15.i2.100373
Diagnostic value of ultrasonography for post-liver transplant hepatic vein complications
Abstract
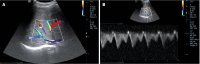
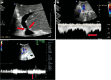
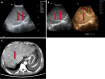
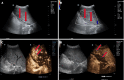
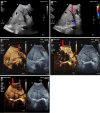
Liver transplantation (LT) is the most effective treatment for patients with end-stage liver disease, and maintaining vascular patency of the transplanted liver is one of the crucial prerequisites for surgical success. Despite hepatic vein complications following LT occurring at a relatively low frequency, ranging between 2% to 11%, delayed diagnosis and treatment may lead to graft dysfunction and even patient mortality. Clinical manifestations of hepatic vein complications are often subtle and nonspecific, posing challenges for early diagnosis. Signs may initially present as mild abnormalities in liver function, delayed recovery of liver function, unexplained ascites, lower limb edema, and perineal edema. Prolonged duration of these complications can lead to hepatic sinusoidal dilatation and eventual liver failure due to prolonged hepatic congestion. Ultrasonography has become the preferred imaging modality for post-liver transplant evaluation due to its convenience and non-invasiveness. Although hepatic vein complications may manifest as disappearance or flattening of the hepatic vein spectrum on routine ultrasound imaging, these findings lack specificity. Contrast-enhanced ultrasound that visualizes the filling of contrast agent in the hepatic veins and dynamically displays blood flow perfusion information in the drainage area can, however, significantly improve diagnostic confidence and provide additional information beyond routine ultrasound examination.
Keywords: Hepatic congestion; Hepatic vein complication; Liver transplantation; Ultrasound; Vascular complication.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflict-of-interest to report.
Figures
Similar articles
-
Isolated Limb Perfusion Can Avert Amputation Indication in Initially Nonsalvageable Sarcomas of the Extremities.Clin Orthop Relat Res. 2025 Jun 19. doi: 10.1097/CORR.0000000000003584. Online ahead of print. Clin Orthop Relat Res. 2025. PMID: 40536544
-
Severe alpha-1 antitrypsin deficiency is associated with a higher risk of complications after first decompensation than other aetiologies of cirrhosis.JHEP Rep. 2025 Mar 20;7(6):101398. doi: 10.1016/j.jhepr.2025.101398. eCollection 2025 Jun. JHEP Rep. 2025. PMID: 40535554 Free PMC article.
-
Platelet Indices and RDW to Assess Inflammatory Milieu in Subclinical Hashimoto's Thyroiditis.Clin Med Insights Endocrinol Diabetes. 2025 Jun 13;18:11795514251349337. doi: 10.1177/11795514251349337. eCollection 2025. Clin Med Insights Endocrinol Diabetes. 2025. PMID: 40528863 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Enhancing the diagnostic potential of electroretinography in Parkinson's disease: A review of protocol and cohort criteria.J Parkinsons Dis. 2025 Jun;15(4):694-709. doi: 10.1177/1877718X251331863. Epub 2025 Apr 29. J Parkinsons Dis. 2025. PMID: 40530583 Review.
References
-
- Galloux A, Pace E, Franchi-Abella S, Branchereau S, Gonzales E, Pariente D. Diagnosis, treatment and outcome of hepatic venous outflow obstruction in paediatric liver transplantation: 24-year experience at a single centre. Pediatr Radiol. 2018;48:667–679. - PubMed
-
- Kosaka T, Eguchi S, Hidaka M, Adachi T, Yoshino K, Kanetaka K, Takatsuki M, Ito S. IVC angioplasty using an autologous vascular graft for IVC stenosis due to metallic stent in a pediatric liver transplant. Pediatr Transplant. 2019;23:e13475. - PubMed
-
- Sambommatsu Y, Hirukawa K, Shimata K, Honda M, Sakurai Y, Ishii M, Ibuki S, Isono K, Irie T, Kawabata S, Hirao H, Sugawara Y, Tamura Y, Ikeda O, Hirai T, Inomata Y, Morinaga J, Hibi T. Hepatic venous outflow obstruction after adult living donor liver transplantation. Liver Transpl. 2023;29:1292–1303. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

