C3 glomerulopathy post kidney transplantation: A single center experience
- PMID: 40535493
- PMCID: PMC11886292
- DOI: 10.5500/wjt.v15.i2.101517
C3 glomerulopathy post kidney transplantation: A single center experience
Abstract
Background: C3 glomerulopathies (C3G) are a rare cause of kidney failure resulting from complement dysregulation. Small studies demonstrate a high rate of recurrence and poor outcomes in kidney transplantation. Treatment efficacy in this setting with eculizumab, a terminal complement inhibitor, is largely unknown.
Aim: To determine the outcomes of kidney transplantation in patients with C3G and the potential impact of eculizumab.
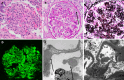
Methods: We retrospectively studied kidney transplant recipients who underwent a post-transplant biopsy confirming C3G between January 1, 1993 and December 31, 2023 at a single center. Only the first episode of kidney transplant was reviewed. The electronic medical records were reviewed for post-transplant allograft function, indication for biopsy, time to biopsy from transplant, time to allograft failure from transplantation, post-C3G treatment, complement laboratory testing, and concurrent malignancy/infection. Reports, and when available slides and immunofluorescence/electron microscopic images, were re-reviewed by a renal pathologist.
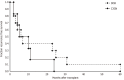
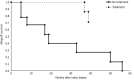
Results: A total of fifteen patients were included in this study. Fourteen patients had suspected recurrent disease, with a pre-transplant native kidney report of C3G. One patient developed de novo C3G. Median post kidney transplant clinical follow up time was 91 months. Median time to recurrence was 7 months with median graft survival of 48 months post kidney transplantation. The most common index biopsy pattern of injury was endocapillary proliferative glomerulonephritis (often with exudative features) with or without mesangial hypercellularity (56%) followed by membranoproliferative glomerulonephritis (25%). Most patients developed membranoproliferative glomerulonephritis pattern of injury on follow up biopsies (63%). Seven patients with recurrent disease received treatment with eculizumab with a median graft survival of 73 months, with five functioning grafts by the end of the study period. Seven patients with recurrent disease did not receive therapy, and all lost their graft with a median graft survival of 22 months (P = 0.003).
Conclusion: C3G following kidney transplantation is mostly a recurrent disorder with a poor prognosis in untreated patients. Untreated recurrence has a poor prognosis with median allograft survival < 2 years. Early treatment with eculizumab may improve transplant outcomes in patients with recurrent C3G.
Keywords: C3 glomerulonephritis; C3 glomerulopathy; Complement disorder; Dense deposit disease; Kidney transplant; Recurrent disease; Renal pathology, Kidney biopsy.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures
Similar articles
-
C3 Glomerulopathy Recurs Early after Kidney Transplantation in Serial Biopsies Performed within the First 2 Years after Transplantation.Clin J Am Soc Nephrol. 2024 Aug 1;19(8):1005-1015. doi: 10.2215/CJN.0000000000000474. Epub 2024 Jun 7. Clin J Am Soc Nephrol. 2024. PMID: 39116277 Free PMC article.
-
C3 Glomerulopathy.2007 Jul 20 [updated 2018 Apr 5]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2007 Jul 20 [updated 2018 Apr 5]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301598 Free Books & Documents. Review.
-
Successful Management of C3 Glomerulopathy Recurrence Post-Kidney Transplantation with Iptacopan: A Case Report.Int J Mol Sci. 2025 May 24;26(11):5053. doi: 10.3390/ijms26115053. Int J Mol Sci. 2025. PMID: 40507864 Free PMC article.
-
Defining the Etiology of Renal Allograft Dysfunction Using Banff 2019 Classification: Correlation with Post-Transplant Duration and Creatinine Levels-A Comprehensive Analysis of 200 Renal Biopsies at a Tertiary Care Medical Center Hospital.Int J Surg Pathol. 2025 May;33(3):615-622. doi: 10.1177/10668969241283737. Epub 2024 Oct 3. Int J Surg Pathol. 2025. PMID: 39360394
-
Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy.Cochrane Database Syst Rev. 2019 Nov 20;2019(11):CD012024. doi: 10.1002/14651858.CD012024.pub3. Cochrane Database Syst Rev. 2019. PMID: 31745984 Free PMC article.
References
-
- Michels MAHM, Wijnsma KL, Kurvers RAJ, Westra D, Schreuder MF, van Wijk JAE, Bouts AHM, Gracchi V, Engels FAPT, Keijzer-Veen MG, Dorresteijn EM, Volokhina EB, van den Heuvel LPWJ, van de Kar NCAJ. Long-term follow-up including extensive complement analysis of a pediatric C3 glomerulopathy cohort. Pediatr Nephrol. 2022;37:601–612. - PMC - PubMed
-
- Pickering MC, D'Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Frémeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodríguez de Córdoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079–1089. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

