Influencing Factors of Adherence to Adjuvant Endocrine Therapy in Breast Cancer Patients: A Meta-Analysis
- PMID: 40535520
- PMCID: PMC12174669
- DOI: 10.1002/hsr2.70934
Influencing Factors of Adherence to Adjuvant Endocrine Therapy in Breast Cancer Patients: A Meta-Analysis
Abstract
Background and aims: Adherence to adjuvant endocrine therapy (AET) in breast cancer patients is affected by multiple factors. A number of studies were performed to find out the potential influencing factors. However, the results of these studies were not consistent. Therefore, this meta-analysis aimed to synthesize the existing evidence on influencing factors of AET adherence in breast cancer patients.
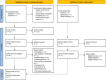
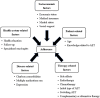
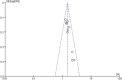
Methods: Several databases were searched for published and unpublished studies. The potential influencing factors were identified and mapped to the five dimensions of the World Health Organization (WHO) medication adherence theoretical framework. Odds ratios (OR) and 95% confidence intervals (95% CI) were used to evaluate the influencing factors, and then meta-analysis was performed using Review Manager 5.4 software.
Results: Thirty-seven eligible studies were included, with a total of 104,777 patients. Nineteen influencing factors were divided into five categories: patient-related factors (OR = 1.74, 95% CI: 1.55-1.96), therapy-related factors (OR = 2.13, 95% CI: 1.85-2.46), disease-related factors (OR = 1.38, 95% CI: 1.25-1.52), socioeconomic factors (OR = 1.34, 95% CI: 1.20-1.50) and health system-related factors (OR = 0.46, 95% CI: 0.26-0.81). Age < 50 years, age > 65 years, lack of knowledge about AET, side effects, without radiotherapy or chemotherapy, initial use of tamoxifen, switching AET, complementary or alternative treatment use, higher Charlson comorbidity index, taking multiple medications, depression, lower income, no medical insurance, no partner, and lack of social support were related to non-adherence. A higher level of medical support was related to good adherence.
Conclusion: AET adherence is influenced by multiple factors. This meta-analysis synthesizes existing evidence on influencing factors for AET adherence in breast cancer patients, offering a comprehensive overview. Governments and stakeholders should align health policy or interventions with the WHO five dimensions of medication adherence to enhance tailored AET adherence strategies.
Keywords: adherence; adjuvant endocrine therapy; breast cancer; influencing factors; meta‐analysis.
© 2025 The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Improving medication adherence to endocrine therapy in breast cancer patients: a mixed-methods systematic review of effective communication strategies for healthcare providers.Breast. 2025 Aug;82:104510. doi: 10.1016/j.breast.2025.104510. Epub 2025 May 28. Breast. 2025. PMID: 40450986 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Prevalence and odds of anxiety and depression in cutaneous malignant melanoma: a proportional meta-analysis and regression.Br J Dermatol. 2024 Jun 20;191(1):24-35. doi: 10.1093/bjd/ljae011. Br J Dermatol. 2024. PMID: 38197404
-
A systematic review of patient-reported outcome measures (PROMs) to assess health-related quality of life (HRQoL) for breast cancer patients who are undertaking adjuvant endocrine therapy.Qual Life Res. 2025 Jun 18. doi: 10.1007/s11136-025-04004-y. Online ahead of print. Qual Life Res. 2025. PMID: 40533702
-
Concurrent factors associated with adherence to adjuvant endocrine therapy among women with non-metastatic breast cancer.J Cancer Surviv. 2025 Aug;19(4):1335-1345. doi: 10.1007/s11764-024-01556-9. Epub 2024 Feb 24. J Cancer Surviv. 2025. PMID: 38401012
References
-
- Cahir C., Bennett K., Dombrowski S. U., et al., “Informing Interventions to Improve Uptake of Adjuvant Endocrine Therapy in Women With Breast Cancer: A Theoretical‐Based Examination of Modifiable Influences on Non‐Adherence,” Supportive Care in Cancer 31, no. 3 (2023): 200, 10.1007/s00520-023-07658-x. - DOI - PMC - PubMed
-
- Cahir C., Guinan E., Dombrowski S. U., Sharp L., and Bennett K., “Identifying the Determinants of Adjuvant Hormonal Therapy Medication Taking Behaviour in Women With Stages I‐III Breast Cancer: A Systematic Review and Meta‐Analysis,” Patient Education and Counseling 98 (2015): 1524–1539, 10.1016/j.pec.2015.05.013. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

