Prospective observational study of tofacitinib in ulcerative colitis - analysis of clinical data, fatigue and health-related quality of life during the induction phase
- PMID: 40535533
- PMCID: PMC12174757
- DOI: 10.1177/17562848251343427
Prospective observational study of tofacitinib in ulcerative colitis - analysis of clinical data, fatigue and health-related quality of life during the induction phase
Abstract
Background: Tofacitinib is a Janus kinase inhibitor for the treatment of ulcerative colitis (UC). Prospective real-world data are scarce.
Objectives: To collect data on clinical outcomes, including health-related quality of life (HRQoL) and fatigue during treatment with tofacitinib.
Design: This is a prospective observational multicentre study in Sweden. In this analysis, outcomes at weeks 2, 8 and 16 are reported.
Methods: Patients with active UC confirmed with endoscopy or faecal calprotectin (FC) were enrolled during 2020-2023 when starting tofacitinib therapy.
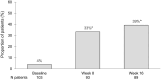
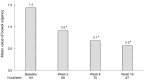
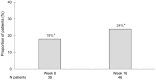
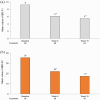
Results: In total, 103 patients were included. After 2 weeks of treatment, 50% (39/78) had achieved symptomatic response and at week 16, 39% (35/89) had achieved corticosteroid-free clinical remission according to the partial Mayo score. At week 16, a reduction in FC by ⩾50% was seen in 49% (35/71) and 24% (11/46) were in endoscopic remission. The frequency of arthralgia decreased from 29% (30/103) at baseline to 11% (10/89) at week 16. Regarding HRQoL at week 16; each of the four Short Health Scale dimensions (symptoms, social function, disease-related worry and general well-being) had improved by a median of 1 point (p < 0.01) and the European Quality of Life 5 Dimensions 5 Levels index improved from 0.80 to 0.87. Finally, the Inflammatory Bowel Disease Fatigue score measuring occurrence and severity showed an improvement with a decrease from 9 points at baseline to 6 at week 16 (p < 0.05).
Conclusion: Induction therapy with tofacitinib therapy was associated with improvements in patient-reported outcome measures of symptoms, endoscopic activity, arthralgia, HRQoL and fatigue. These real-world data illustrate that tofacitinib is a fast-acting drug with broad therapeutic effects in UC.
Clinicaltrial registration number: NCT04338204.
Keywords: JAK inhibitor; fatigue; health-related quality of life; tofacitinib; ulcerative colitis.
Plain language summary
Tofacitinib treatment in ulcerative colitis; results from treatment in the real world What do we hope to find out? If patients with Ulcerative Colitis and treatment with tofacitinib improve in various expressions of their disease. Why was this research done? Ulcerative colitis is an inflammatory condition of the bowel that often affects a person’s life substantially with an impact on quality of life. Symptoms of the bowel such as diarrhea, blood in stool and urgency are limiting symptoms but fatigue and joint pain may also be present. Some of the symptoms may be independent to intestinal inflammation. Before approval, drugs must demonstrate effectiveness in intestinal inflammation in randomized trials, but treatment and results in a clinical reality may differ from these settings. What did we do? Patients with active ulcerative colitis suitable for tofacitinib treatment were included in the study. Information concerning bowel symptoms, laboratory tests, quality of life and fatigue were collected at inclusion, week 2, week 8 and week 16. For some patients, endoscopic data was available. Information at follow up were compared to data before treatment for the same patient. Established questionnaires were used to quantify and evaluate the patient reported parameters. What did we find out? From 16 hospitals in Sweden, 103 patients were included. During the first 16 week of treatment, patients improved in symptoms. At week 16, 4/10 patients were in clinical remission. Quality of life improved in all measured aspects. Occurrence and severity of fatigue decreased by 1/3. Patients reporting joint pain at inclusion decreased to 1/3 at week 16. Some improvements were seen already week 2. These results indicate broad effects of tofacitinib.
© The Author(s), 2025.
Conflict of interest statement
H.S. has served as a speaker and/or advisory board member for AbbVie, Takeda, Janssen, Pfizer, Gilead, MSD, Tillotts, Pharmacosmos and Ferring. O.O. has been PI on projects at Karolinska Institutet financed by grants from Janssen, Pfizer, AbbVie, Takeda, Ferring, Galapagos/Alfasigma and Bristol-Myers Squibb. Karolinska Institutet has also received fees for lectures and participation by O.O. on advisory boards from Janssen, Ferring, Galapagos, Bristol-Myers Squibb, Takeda and Pfizer. C.R.H.H. has served as a speaker and/or advisory board member for Takeda, Galapagos, Ferring, AbbVie, Janssen and Pfizer. She has received grants from Takeda and Tillotts. J.H. has served as speaker and/or advisory board member for AbbVie, Aqilion, BMS, Celgene, Celltrion, Dr Falk Pharma and the Falk Foundation, Eli Lilly, Ferring, Galapagos, Gilead, Hospira, Index Pharma, Janssen, MEDA, Medivir, Medtronic, MSD, Novartis, Pfizer, Prometheus Laboratories Inc., Sandoz, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, UCB and received grant support from Janssen, MSD and Takeda. J.M. has served as a speaker and/or advisory board member for AbbVie, Bayer, Bristol-Myers Squibb, Galapagos, Hospira, Janssen, MSD, Pfizer, Sandoz, Takeda and UCB Pharma, and has received grant support from AbbVie, Fresenius Kabi, Pfizer and Takeda. O.G. has served as speaker and/or advisory board member for AbbVie, Bristol-Myers Squibb, Ferring, Janssen-Cilag, MSD, Pfizer, Takeda, Tillotts Pharma, Vifor Pharma. H.H. has been PI on projects at Linköping University financed by grants from AbbVie, Ferring, Takeda and Tillotts. H.H. has also received fees for lectures and participation on advisory boards from AbbVie, Fresenius Kabi, Janssen, Norgine, Pfizer, Pharmacosmos, Takeda and Tillotts. L.N. has served as a speaker for AbbVie and Mylan and participated on the advisory board for Takeda. S.J. has served as a speaker and/or advisory board for Takeda, Eli Lilly, Bristol-Myers Squibb, AbbVie, Galapagos, Vifor Pharma. M.S. and D.H. are former employees of Pfizer, and own stocks in the company. J.C.C. is an employee of Pfizer and owns stock in the company.
Figures


References
-
- Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis 2022; 16(1): 2–17. - PubMed
-
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160(5): 1570–1583. - PubMed
-
- Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376(18): 1723–1736. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

