Construction of a genome-engineered stable 5-aminolevulinic acid producing Corynebacterium glutamicum by increasing succinyl-CoA supply
- PMID: 40535562
- PMCID: PMC12173734
- DOI: 10.1016/j.synbio.2025.05.013
Construction of a genome-engineered stable 5-aminolevulinic acid producing Corynebacterium glutamicum by increasing succinyl-CoA supply
Abstract
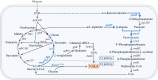
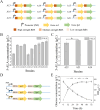
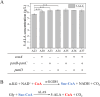
5-Aminolevulinic acid (5-ALA), a versatile precursor for tetrapyrrole derivatives (such as heme, chlorophyll, and cobalamin), drives advancing microbial cell factories to meet growing biomedical and industrial demands. However, there remain two challenges that limit yield and scalability: the limitations of conventional plasmid-based gene expression systems and the lack of fine regulation of succinyl-CoA. In this study, to address these limitations, we integrated multiple copies of hemA C132A of the heterologous C4 pathway on the genome. For fine regulating the supply of succinyl-CoA, the genes related to the tricarboxylic acid cycle (TCA cycle) oxidation branch pathway were combinatorially screened. The optimal combination of icd and lpd was confirmed by ribosome binding site (RBS) engineering, which was integrated on the genome with optimized expression intensity. Succinyl-CoA supply was further increased by genome integration and expression optimization of key CoA biosynthetic gene coaA, pantothenic acid synthesis-related gene panB-panC, and β-alanine synthesis-related gene panD. The optimized genomically stable chassis achieved a high 5-ALA production of 6.38 ± 0.16 g/L, which was 8.63-fold higher than the single hemA C132A copy strain A1 (0.74 ± 0.07 g/L). From these findings, a stable and high-yield 5-ALA synthetic strain was successfully constructed, providing a new strategy for production of biochemicals derived from succinyl-CoA in C. glutamicum.
Keywords: 5-Aminolevulinic acid; C4 pathway; Corynebacterium glutamicum; Succinyl-CoA.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Metabolic engineering of Escherichia coli for high-yield dopamine production via optimized fermentation strategies.Appl Environ Microbiol. 2025 Jun 18;91(6):e0015925. doi: 10.1128/aem.00159-25. Epub 2025 May 8. Appl Environ Microbiol. 2025. PMID: 40338089 Free PMC article.
-
Construction of Bacillus velezensis engineering strains for producing 5-aminolevulinic acid to elicit plant resistance against abiotic stresses and tobacco bacterial wilt disease.Pest Manag Sci. 2025 Sep;81(9):5602-5613. doi: 10.1002/ps.8915. Epub 2025 May 15. Pest Manag Sci. 2025. PMID: 40375689
-
Metabolic Engineering of Corynebacterium glutamicum for the Fermentative Production of Gallic Compounds by Extending the Shikimate Pathway.J Microbiol Biotechnol. 2025 Jun 12;35:e2504009. doi: 10.4014/jmb.2409.04009. J Microbiol Biotechnol. 2025. PMID: 40537910 Free PMC article.
-
Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy.Cochrane Database Syst Rev. 2019 Nov 20;2019(11):CD012024. doi: 10.1002/14651858.CD012024.pub3. Cochrane Database Syst Rev. 2019. PMID: 31745984 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
-
- Chen J.Z., Wang Y., Pu W., Zheng P., Sun J.B. Advances and perspective on bioproduction of 5-aminolevulinic acid. Synbio J. 2021;2:1000–1016. doi: 10.12211/2096-8280.2021-010. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

