Platinum(IV) anticancer therapies and cathepsin B: innovative strategies for overcoming resistance in glioblastoma cells
- PMID: 40535571
- PMCID: PMC12174597
- DOI: 10.3389/fcell.2025.1506206
Platinum(IV) anticancer therapies and cathepsin B: innovative strategies for overcoming resistance in glioblastoma cells
Abstract
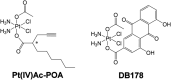
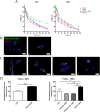
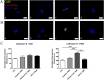
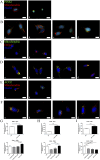
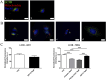
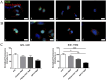
Glioblastoma (GBM) is the most frequent and aggressive brain tumor in adults. Due to its heterogeneity, the abundance of altered signaling pathways within the same tumoral mass, its low immunogenicity, and the presence of the blood-brain barrier, standard therapies based on surgical resection, radiotherapy, and chemotherapy result in ineffective tumor removal. For these reasons, the development of new drugs is mandatory to ameliorate patients' life expectancy and quality of life. Cathepsins are lysosomal proteases involved in several physiological and pathological processes, and they play key roles in modulating cell death and pharmacological resistance. In particular, cathepsin B is a crucial regulatory protein in different types of cell death, and its overexpression contributes to GBM angiogenesis and tumor progression. Octahedral platinum(IV) (Pt(IV))-based prodrugs have already demonstrated improved anticancer efficacy compared to routinely used cisplatin. This work aims to investigate the effects of two such prodrugs-Pt(IV)Ac-POA ((OC-6-44)-acetatodiamminedichlorido(2-(2-propynyl)octanoato)platinum(IV)) and DB178 ((OC-6-44)-acetatodiamminedichlorido(4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylato)platinum(IV))-on two different glioblastoma cell lines, U251 and T98G, with particular attention to their effects on cathepsin B. The immunocytochemical and biochemical results obtained on the two cell lines highlight the maintenance of basal levels of cathepsin B while efficiently activating programmed cell death mechanisms, as investigated by optical and electronic microscopy. These findings may serve as a valid starting point for further approaches that incorporate cathepsins' inhibitors to improve therapeutic efficacy and possibly reveal novel pharmacological targets.
Keywords: apoptosis; cathepsin B; drug resistance; glioblastoma; mitophagy; platinum(IV).
Copyright © 2025 Casali, Gaiaschi, Pelloni, Gola, Cavallo, Milanesi, Ravera, Biggiogera, De Luca and Bottone.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer AP declared a shared affiliation with the author FDL to the handling editor at the time of review.
Figures


References
-
- Astesana V., Faris P., Ferrari B., Siciliani S., Lim D., Biggiogera M., et al. (2021). [Pt(O,O’-acac)(γ-acac)(DMS)]: alternative strategies to overcome cisplatin-induced side effects and resistance in T98G glioma cells. Cell Mol. Neurobiol. 41, 563–587. 10.1007/s10571-020-00873-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

