Comparison of survival benefit and safety profile between lenvatinib and donafenib as conversion therapy in patients with hepatocellular carcinoma
- PMID: 40535653
- PMCID: PMC12170375
- DOI: 10.62347/PBLA2928
Comparison of survival benefit and safety profile between lenvatinib and donafenib as conversion therapy in patients with hepatocellular carcinoma
Abstract
Objective: To compare the survival benefit and safety profiles between lenvatinib and donafenib when used as conversion therapies for patients with hepatocellular carcinoma (HCC) at the China National Liver Cancer (CNLC) stages I-III.
Methods: A retrospective comparative study was conducted on 76 patients diagnosed with HCC at CNLC stage I-III. Among them, 40 patients were treated with lenvatinib, and the other 36 patients received donafenib. Key outcomes, including overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and adverse events, were evaluated.
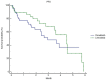
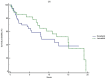
Results: Patients treated with lenvatinib showed significantly longer OS (14.9 vs. 7.9 months, P=0.010) and PFS (4.6 vs. 2.9 months, P<0.001) compared to those treated with donafenib. The ORR was 15% in the lenvatinib group and 5.6% in the donafenib group (P=0.551). Lenvatinib was also associated with a lower incidence of grade ≥3 adverse events (P<0.05). Specifically, severe adverse events such as hepatotoxicity, hematological toxicity, hand-foot syndrome, and diarrhea were less frequent in the lenvatinib cohort. Univariate and multivariate analyses identified elevated alpha-fetoprotein (AFP) levels and the presence of hepatic vein tumor thrombus as significant predictors of poorer PFS, with hazard ratios (HR) of 1.45 and 1.80, respectively. Furthermore, multivariate analysis revealed that higher Child-Pugh scores and elevated AFP levels were associated with worse OS (all P<0.05).
Conclusion: Lenvatinib demonstrates superior survival outcomes compared to donafenib as a conversion therapy in patients with CNLC stage I-III HCC. While the two therapies are comparable in overall safety profiles, lenvatinib is more tolerated, with a lower incidence of severe adverse events.
Keywords: Hepatocellular carcinoma; conversion therapy; donafenib; lenvatinib; survival benefit.
AJTR Copyright © 2025.
Conflict of interest statement
None.
Figures
References
-
- Ganesan P, Kulik LM. Hepatocellular carcinoma: new developments. Clin Liver Dis. 2023;27:85–102. - PubMed
-
- Wang L, Zhu L, Liang C, Huang X, Liu Z, Huo J, Zhang Y, Zhang Y, Chen L, Xu H, Li X, Xu L, Kuang M, Wong CC, Yu J. Targeting N6-methyladenosine reader YTHDF1 with siRNA boosts antitumor immunity in NASH-HCC by inhibiting EZH2-IL-6 axis. J Hepatol. 2023;79:1185–1200. - PubMed
-
- Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629–652. - PubMed
-
- Alawyia B, Constantinou C. Hepatocellular carcinoma: a narrative review on current knowledge and future prospects. Curr Treat Options Oncol. 2023;24:711–724. - PubMed
LinkOut - more resources
Full Text Sources