Evaluation of the combined use of linaclotide and probiotics on clinical treatment efficacy and quality of life in patients with constipation-predominant irritable bowel syndrome
- PMID: 40535661
- PMCID: PMC12170367
- DOI: 10.62347/GNSB4367
Evaluation of the combined use of linaclotide and probiotics on clinical treatment efficacy and quality of life in patients with constipation-predominant irritable bowel syndrome
Abstract
Objective: To evaluate the effects of combining linaclotide with Bifid Triple Viable Capsules on clinical outcomes and quality of life (QoL) in patients with constipation-predominant irritable bowel syndrome (IBS-C).
Methods: A retrospective analysis was performed on data from 189 IBS-C patients treated between April 2021 and January 2024. The control group (91 patients) received linaclotide, while the combined group (98 patients) received linaclotide plus Bifid Triple Viable Capsules. Outcomes assessed included bowel movement frequency, stool consistency scores, constipation severity, anxiety (Self-Rating Anxiety Scale, SAS), depression (Self-Rating Depression Scale, SDS), QoL (Irritable Bowel Syndrome Quality of Life, IBS-QoL), and symptom severity (Irritable Bowel Syndrome Severity Scoring System, IBS-SSS). Logistic regression identified independent risk factors for QoL improvement.
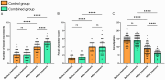
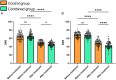
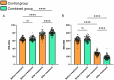
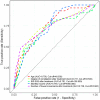
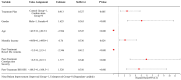
Results: Both groups showed significant increases in bowel movement frequency after treatment (P < 0.001). The combined group experienced a significantly greater improvement compared to the control group (P < 0.001). Stool consistency scores improved significantly in both groups (P < 0.001), but no significant difference was observed between groups (P > 0.05). Both groups showed significant reductions in constipation severity, with the combined group showing greater improvement (P < 0.001). SAS and SDS scores decreased significantly in both groups (P < 0.001). The combined group showed greater reductions in SAS (P < 0.05) and SDS (P < 0.001). IBS-QoL scores improved significantly in both groups, with the combined group achieving greater improvement (P < 0.001). IBS-SSS scores decreased significantly, with the combined group experiencing a greater reduction (P < 0.001). IBS-QoL scores were positively correlated with bowel movement frequency (r = 0.289, P < 0.001) and negatively correlated with stool consistency scores (r = -0.154, P = 0.036), constipation severity (r = -0.386, P < 0.001), SDS scores (r = -0.150, P = 0.040), and IBS-SSS scores (r = -0.347, P < 0.001). Logistic regression identified treatment regimen (OR = 0.163, P = 0.017), age (OR = 4.666, P = 0.002), monthly income (OR = 0.065, P < 0.001), post-treatment bowel movement frequency (OR = 0.055, P < 0.001), and post-treatment constipation severity (OR = 5.545, P = 0.007) as independent factors influencing QoL improvement.
Conclusion: The combined use of linaclotide and Bifid Triple Viable Capsules significantly enhances bowel movement frequency, reduces constipation severity, and improves QoL and psychological well-being in IBS-C patients. This approach offers a promising strategy for the comprehensive management of IBS-C.
Keywords: Constipation-predominant irritable bowel syndrome; clinical efficacy; linaclotide; probiotics; quality of life.
AJTR Copyright © 2025.
Conflict of interest statement
None.
Figures


Similar articles
-
Tegaserod for the treatment of irritable bowel syndrome and chronic constipation.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD003960. doi: 10.1002/14651858.CD003960.pub3. Cochrane Database Syst Rev. 2007. PMID: 17943807
-
Effect of linaclotide in irritable bowel syndrome with constipation (IBS-C): a systematic review and meta-analysis.Neurogastroenterol Motil. 2014 Apr;26(4):499-509. doi: 10.1111/nmo.12292. Epub 2013 Dec 18. Neurogastroenterol Motil. 2014. PMID: 24351035
-
A Multicenter Randomized Controlled Trial of Microbiome-Based Artificial Intelligence-Assisted Personalized Diet vs Low-Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet: A Novel Approach for the Management of Irritable Bowel Syndrome.Am J Gastroenterol. 2024 Sep 1;119(9):1901-1912. doi: 10.14309/ajg.0000000000002862. Epub 2024 May 8. Am J Gastroenterol. 2024. PMID: 38717025 Free PMC article. Clinical Trial.
-
Physical activity for treatment of irritable bowel syndrome.Cochrane Database Syst Rev. 2022 Jun 29;6(6):CD011497. doi: 10.1002/14651858.CD011497.pub2. Cochrane Database Syst Rev. 2022. PMID: 35766861 Free PMC article.
-
Randomised controlled trial: nutritional supplements to relieve irritable bowel syndrome symptoms by targeting the gut microbiota.J Nutr Sci. 2025 Jul 11;14:e46. doi: 10.1017/jns.2025.10021. eCollection 2025. J Nutr Sci. 2025. PMID: 40692549 Free PMC article. Clinical Trial.
References
-
- Zhou J, Wei H, Zhou A, Xiao X, Xie X, Tang B, Lin H, Tang L, Meng R, Yuan X, Zhang J, Huang C, Huang B, Liao X, Zhong T, He S, Gu S, Yang S. The gut microbiota participates in the effect of linaclotide in patients with irritable bowel syndrome with constipation (IBS-C): a multicenter, prospective, pre-post study. J Transl Med. 2024;22:98. - PMC - PubMed
-
- Curtis S, Curtis M. Tenapanor (Ibsrela) for the treatment of irritable bowel syndrome with constipation. Am Fam Physician. 2022;105:656–658. - PubMed
-
- Tack J, Talley NJ. Gastroduodenal disorders. Am J Gastroenterol. 2010;105:757–763. - PubMed
-
- Xing Z, Hou X, Zhou K, Qin D, Pan W. Impact of parental-rearing styles on irritable bowel syndrome in adolescents: a school-based study. J Gastroenterol Hepatol. 2014;29:463–468. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
