Bioengineered platelet nanoplatform enables renal-targeted dexamethasone delivery for chronic nephritis therapy with dual anti-inflammatory/anti-fibrotic effects and minimized systemic toxicity
- PMID: 40535704
- PMCID: PMC12173822
- DOI: 10.1016/j.bioactmat.2025.06.002
Bioengineered platelet nanoplatform enables renal-targeted dexamethasone delivery for chronic nephritis therapy with dual anti-inflammatory/anti-fibrotic effects and minimized systemic toxicity
Abstract
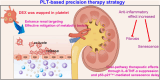
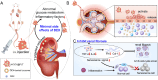
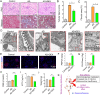
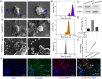
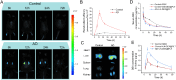
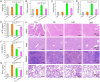
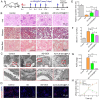
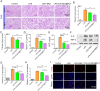
Chronic nephritis management remains challenging due to the compromised therapeutic efficacy and severe systemic complications of conventional glucocorticoid therapy. Here, we developed a bioinspired platelet-mediated delivery system (LN-DEX@PLT) that leverages platelet tropism toward injured glomeruli for precision drug delivery. This system integrates lipid nanoemulsion encapsulation with platelet-mediated hitchhiking delivery to achieve three key functionalities: (1) enhanced renal targeting demonstrated by 2.2-fold higher glomerular accumulation compared to free dexamethasone via In vivo imaging, (2) effective mitigation of glucocorticoid-induced metabolic toxicity evidenced by reduced fasting plasma glucose (5.2 ± 0.3 vs 8.3 ± 0.7 mmol/L in free DEX), suppression of hepatic gluconeogenic enzymes (PEPCK expression decreased by 43 %, G-6 Pase by 51 %, both p < 0.001), and suppressed body weight (-23.1 % versus free DEX group), and (3) dual-pathway therapeutic effects through IL-6/TNF-α suppression and p53-p21Cip1-mediated senescence delay. In Adriamycin-based chronic nephritis models, LN-DEX@PLT demonstrated superior renal protection with 81 % reduction in proteinuria (vs 33 % for free DEX). In LPS-induced and Adriamycin-based chronic nephritis models, LN-DEX@PLT demonstrated suppression of renal inflammation markers (IL-6 expression decreased to 68 %, TNF-α to 51 %) and macrophage infiltration (F4/80+ cells decreased 5.3-fold). This platelet-biohybrid system provides a clinically translatable paradigm for precision glucocorticoid therapy with reduced dosing frequency.
Keywords: Chronic nephritis; Dexamethasone; Minimized senescence effects; Platelets; Precision therapy.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Dexamethasone disrupts intracellular pH homeostasis to delay coronavirus infectious bronchitis virus cell entry via sodium hydrogen exchanger 3 activation.J Virol. 2025 Jun 17;99(6):e0189424. doi: 10.1128/jvi.01894-24. Epub 2025 May 9. J Virol. 2025. PMID: 40340398 Free PMC article.
-
Platelet Indices and RDW to Assess Inflammatory Milieu in Subclinical Hashimoto's Thyroiditis.Clin Med Insights Endocrinol Diabetes. 2025 Jun 13;18:11795514251349337. doi: 10.1177/11795514251349337. eCollection 2025. Clin Med Insights Endocrinol Diabetes. 2025. PMID: 40528863 Free PMC article.
-
Synergistic ROS/enzyme dual-responsive oral drug delivery system: A novel multi-mechanistic platform for spatiotemporal control and overcoming drug resistance in colorectal cancer therapy.Mater Today Bio. 2025 May 30;33:101920. doi: 10.1016/j.mtbio.2025.101920. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40528838 Free PMC article.
-
A Systematic Review of Anti-TNF and Anti-IL-6 Treatments for Pediatric Takayasu Arteritis: Addressing a Therapeutic Dilemma.Paediatr Drugs. 2025 Jun 18. doi: 10.1007/s40272-025-00706-5. Online ahead of print. Paediatr Drugs. 2025. PMID: 40531440
-
Defining disease severity in atopic dermatitis and psoriasis for the application to biomarker research: an interdisciplinary perspective.Br J Dermatol. 2024 Jun 20;191(1):14-23. doi: 10.1093/bjd/ljae080. Br J Dermatol. 2024. PMID: 38419411 Free PMC article. Review.
References
-
- Mühlig A.K., Steingröver J., Heidelbach H.S., Wingerath M., Sachs W., Hermans-Borgmeyer I., Meyer-Schwesinger C., Choi H.Y., Lim B.J., Patry C., Hoffmann G.F., Endlich N., Bracke K., Weiß M., Guse A.H., Lassé M., Rinschen M.M., Braun F., Huber T.B., Puelles V.G., Schmitt C.P., Oh J. The calcium-sensing receptor stabilizes podocyte function in proteinuric humans and mice. Kidney Int. 2022;101:1186–1199. doi: 10.1016/j.kint.2022.01.036. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

