Pore engineering in metal-organic frameworks and covalent organic frameworks: strategies and applications
- PMID: 40535720
- PMCID: PMC12172060
- DOI: 10.1039/d5sc01635e
Pore engineering in metal-organic frameworks and covalent organic frameworks: strategies and applications
Abstract
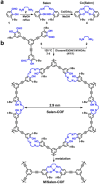
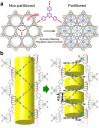
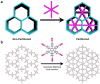
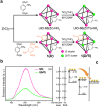
Crystalline porous materials, particularly metal-organic frameworks (MOFs) and covalent organic frameworks (COFs), have garnered significant attention for advanced applications due to their tunable pore environments and versatile functionalities. By precisely controlling factors such as size, shape, functional sites, and pore distribution, MOFs and COFs can be tailored to exhibit high selectivity for specific molecules, making them ideal for applications in gas storage and separation, catalysis, and water remediation. This review provides a background overview, beginning with an introduction to pore surface engineering strategies and the design features of MOFs and COFs. It then highlights recent advancements in three key research areas that our group has investigated in-depth over the past decade, discussing the strategies and principles involved. Finally, we outline the remaining challenges and offer our perspectives on future opportunities for pore-engineered MOFs and COFs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures













References
-
- Li Y. Li L. Yu J. Applications of Zeolites in Sustainable Chemistry. Chem. 2017;3:928–949.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

