Ursolic Acid induces ferroptosis by affecting redox balance and FADS2-mediated unsaturated fatty acid synthesis in Non-Small Cell Lung Cancer
- PMID: 40535804
- PMCID: PMC12170995
- DOI: 10.7150/jca.105863
Ursolic Acid induces ferroptosis by affecting redox balance and FADS2-mediated unsaturated fatty acid synthesis in Non-Small Cell Lung Cancer
Abstract
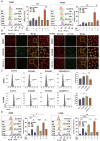
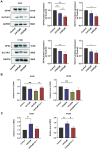
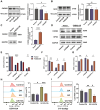
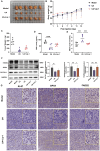
Ursolic Acid (UA) is a naturally occurring pentacyclic triterpenoid compound that is prevalent in various medicinal plants and fruits. It has garnered significant attention due to its broad spectrum of anticancer properties. In this study, we evaluated the antitumor effects of UA on Non-Small Cell Lung Cancer (NSCLC).UA significantly inhibited NSCLC viability and induced cell death in a time- and dose-dependent manner. Furthermore, the administration of UA resulted in an elevation of intracellular reactive oxygen species (ROS), lipid ROS, and ferrous iron levels, while concurrently suppressing the expression of SLC7A11, glutathione, and GPX4. Consequently, this led to an augmentation in the concentration of the lipid peroxidation substrate, malondialdehyde. All the changes were effectively attenuated by the ferroptosis inhibitor Ferrostatin-1(Fer-1) and Deferoxamine (DFO). Moreover, similar observations were made in animal experiments. The sequencing data indicate that UA influences ferroptosis by modulating Fatty Acid Desaturase-2 (FADS2). The reintroduction of FADS2 through ectopic expression restored the resistance to ferroptosis induced by UA in A549 cells, while the addition of exogenous oleic acid (OA) counteracted the impact of UA on the oxidative response. These results suggest that UA induces ferroptosis in NSCLC by affecting redox pathways and the FADS2-mediated synthesis of unsaturated fatty acids.These studies collectively underscore the promising role of UA in the development of effective anticancer therapies.
Keywords: FADS2; Ferroptosis; Redox Regulation; Unsaturated Fatty Acids; Ursolic Acid.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–54. - PubMed
-
- Dai C, Chen X, Li J, Comish P, Kang R, Tang D. Transcription factors in ferroptotic cell death. Cancer Gene Ther. 2020;27:645–56. - PubMed
-
- Yu K, Chen Y, Zhang L. et al. Cancer-Erythrocyte Membrane-Mimicking Fe3O4 Nanoparticles and DHJS for Ferroptosis/Immunotherapy Synergism in Tumors. ACS Appl Mater Interfaces. 2023;15:44689–710. - PubMed
LinkOut - more resources
Full Text Sources

