Theaflavin-3,3'-digallate triggers apoptosis in osteosarcoma cells via the caspase pathway
- PMID: 40535821
- PMCID: PMC12171003
- DOI: 10.7150/jca.111718
Theaflavin-3,3'-digallate triggers apoptosis in osteosarcoma cells via the caspase pathway
Abstract
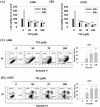
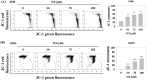
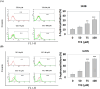
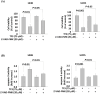
Osteosarcoma is a cancer associated with a guarded prognosis. Various compounds can induce apoptosis in osteosarcoma cells. Theaflavin-3,3'-digallate (TF3) has been demonstrated to alter cell growth and induce apoptosis in various cancer cells. The present study investigated the apoptotic effect of TF3 on osteosarcoma cells. It further explored key apoptotic pathways activated by TF3. Viability of 143B and U2OS osteosarcoma cells after TF3 treatment was assessed. The effects of TF3 on key apoptotic pathways were analyzed. Furthermore, a xenograft mouse model of osteosarcoma was established for in vivo experiments. The results indicated that TF3 significantly reduced the viability of 143B and U2OS cells. Western blotting revealed that TF3 upregulated the expression of cleaved caspase-3 and cleaved caspase-9 in osteosarcoma cells. In addition, TF3 increased the levels of phosphorylated histone H2Ax, Bax, Bak1, and cytochrome c, while reducing the levels of Mcl-1 and survivin in osteosarcoma cells. Furthermore, TF3 significantly reduced the average tumor volume in the xenograft model. Overall, this study suggests that TF3 induces apoptosis in osteosarcoma cells, primarily by regulating the caspase pathway.
Keywords: apoptosis; caspase; osteosarcoma; reactive oxygen species; theaflavin-3,3'-digallate.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–5. - PubMed
-
- Lu KH, Lu EW, Lin CW, Yang JS, Yang SF. New insights into molecular and cellular mechanisms of zoledronate in human osteosarcoma. Pharmacol Ther. 2020;214:107611. - PubMed
-
- Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–18. - PubMed
-
- Lu KH, Lin CW, Hsieh YH, Su SC, Reiter RJ, Yang SF. New insights into antimetastatic signaling pathways of melatonin in skeletomuscular sarcoma of childhood and adolescence. Cancer Metastasis Rev. 2020;39:303–20. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

