The Role of Five-Membered Aromatic Rings Containing N and O in Modulating Bile Acid Receptors: An Overview
- PMID: 40536013
- PMCID: PMC12368480
- DOI: 10.1002/cmdc.202500405
The Role of Five-Membered Aromatic Rings Containing N and O in Modulating Bile Acid Receptors: An Overview
Abstract
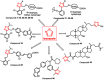
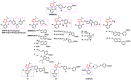
Over the past decades, extensive scientific research in the fields of chemistry and pharmaceutical chemistry has led to the synthesis and study of numerous chemical compounds with diverse therapeutic applications. Many of these compounds feature heterocyclic aromatic structures, including four-, five-, and six-membered rings. Among them, five-membered heteroaromatic rings have garnered particular attention in medicinal chemistry due to their favorable properties, such as enhanced metabolic stability, solubility, and bioavailability, key attributes for the development of effective drugs. The distinctive physicochemical properties and biological activities of five-membered heterocycles have established them as vital structural motifs in numerous clinically effective drugs. These heterocyclic compounds play a crucial role in the design of therapeutic agents, including those targeting bile acid receptors. Bile acid receptor modulators, activated by endogenous bile acids, offer promising potential in treating a variety of metabolic and enterohepatic disorders, such as dyslipidemia, diabetes, cholestasis, and inflammatory bowel disease. This review aims to provide an up-to-date overview of aromatic five-membered nitrogen- and oxygen-containing heterocycles, focusing on their role as bile acid receptor modulators, particularly farnesoid X receptor and G protein-coupled bile acid receptor 1. These receptors are clinically validated targets for the treatment of metabolic disorders and nonalcoholic steatohepatitis.
Keywords: G protein‐coupled bile acid receptors 1; farnesoid X receptors; five‐membered rings; heterocyclic compounds; isoxazole.
© 2025 The Author(s). ChemMedChem published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





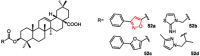
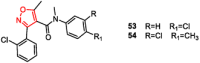





Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Synthetic Approaches and Biological Significance of Four-Membered Heterocyclic Compounds.Curr Top Med Chem. 2025 Jul 23. doi: 10.2174/0115680266375132250715112753. Online ahead of print. Curr Top Med Chem. 2025. PMID: 40708505
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Caffeoylquinic acids from Silphium perfoliatum L. show hepatoprotective effects on cholestatic mice by regulating enterohepatic circulation of bile acids.J Ethnopharmacol. 2025 Jan 30;337(Pt 2):118870. doi: 10.1016/j.jep.2024.118870. Epub 2024 Sep 30. J Ethnopharmacol. 2025. PMID: 39357582
References
-
- Kunied T., Mutsanga H., Palmer, B 2002, 175.
-
- Arora P., Arora V., Lamba H. S., Wadhwa D., Int. J. Pharm. Sci. Res. 2012, 3, 2947.
-
- Garcıa‐Valverde M., Torroba T., J. Investig. Dermatology Symp. Proc. 2001, 6, 188.
-
- Balaban A. T., Oniciu D. C., Chem. Rev. 2004, 104, 2777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

