Salmonella exploits host- and bacterial-derived β-alanine for replication inside host macrophages
- PMID: 40536105
- PMCID: PMC12178601
- DOI: 10.7554/eLife.103714
Salmonella exploits host- and bacterial-derived β-alanine for replication inside host macrophages
Abstract
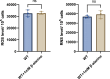
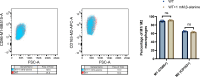
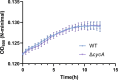
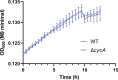
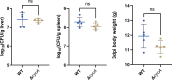
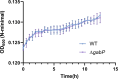
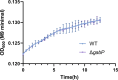
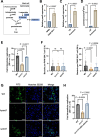
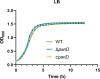
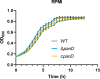
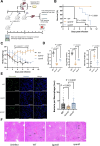
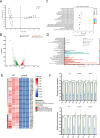
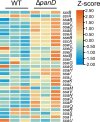
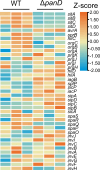
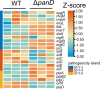
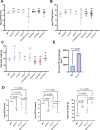
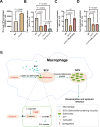
Salmonella is a major foodborne pathogen that can effectively replicate inside host macrophages to establish life-threatening systemic infections. Salmonella must utilize diverse nutrients for growth in nutrient-poor macrophages, but which nutrients are required for intracellular Salmonella growth is largely unknown. Here, we found that either acquisition from the host or de novo synthesis of a nonprotein amino acid, β-alanine, is critical for Salmonella replication inside macrophages. The concentration of β-alanine is decreased in Salmonella-infected macrophages, while the addition of exogenous β-alanine enhances Salmonella replication in macrophages, suggesting that Salmonella can uptake host-derived β-alanine for intracellular growth. Moreover, the expression of panD, the rate-limiting gene required for β-alanine synthesis in Salmonella, is upregulated when Salmonella enters macrophages. Mutation of panD impaired Salmonella replication in macrophages and colonization in the mouse liver and spleen, indicating that de novo synthesis of β-alanine is essential for intracellular Salmonella growth and systemic infection. Additionally, we revealed that β-alanine influences Salmonella intracellular replication and in vivo virulence partially by increasing expression of the zinc transporter genes znuABC, which in turn facilitates the uptake of the essential micronutrient zinc by Salmonella. Taken together, these findings highlight the important role of β-alanine in the intracellular replication and virulence of Salmonella, and panD is a promising target for controlling systemic Salmonella infection.
Keywords: Salmonella; infectious disease; microbiology; replication in macrophage; virulence; zinc uptake; β-alanine.
Plain language summary
Salmonella, a type of bacterium, is one of the most common foodborne pathogens and is responsible for illnesses ranging from gastroenteritis to typhoid fever. Each year, it infects over 100 million people globally, leading to 350,000 deaths. Immune cells called macrophages are important for defending against bacterial infections as they can engulf and destroy harmful bacteria. However, Salmonella is able to survive and multiply within these very immune cells that are meant to eliminate it. To do so, the bacteria require nutrients such as amino acids, which are the building blocks of proteins. These are either produced by the bacteria or obtained from the infected host. However, the specific nutrients that Salmonella require to survive and multiply, as well as their source, remained unknown. To investigate, Ma, Yang et al. measured amino acid levels in macrophages that had been infected with Salmonella and compared them to those in uninfected macrophages. This revealed that the levels of an amino acid called β-alanine – which differs from many amino acids because it is not used to make proteins – are lower in infected macrophages. Furthermore, providing infected macrophages with more β-alanine increased bacterial replication. This suggests that the bacteria acquire this amino acid from the macrophages in order to survive and replicate. To determine whether Salmonella can also make β-alanine themselves, Ma et al. prevented them from producing it, which slowed bacterial growth and led to milder infections in mice. This ability to produce β-alanine required a gene known as PanD. Further experiments also showed that β-alanine assists Salmonella in acquiring the essential micronutrient zinc from macrophages. Taken together, the findings of Ma et al. reveal the critical role of β-alanine in Salmonella growth within macrophages and its ability to cause disease. The panD gene that enables Salmonella to synthesize β-alanine could serve as a potential target for new treatments or vaccines. By targeting this specific aspect of Salmonella's survival strategy, researchers may be able to develop more effective methods to prevent and treat these dangerous infections.
© 2024, Ma, Yang et al.
Conflict of interest statement
SM, BY, YS, XW, HG, RL, TY, CK, JC, LJ No competing interests declared
Figures


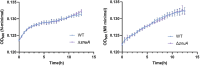
Update of
- doi: 10.1101/2024.10.07.616983
- doi: 10.7554/eLife.103714.1
- doi: 10.7554/eLife.103714.2
- doi: 10.7554/eLife.103714.3
References
-
- Ammendola S, Pasquali P, Pistoia C, Petrucci P, Petrarca P, Rotilio G, Battistoni A. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infection and Immunity. 2007;75:5867–5876. doi: 10.1128/IAI.00559-07. - DOI - PMC - PubMed
-
- Basavanna S, Chimalapati S, Maqbool A, Rubbo B, Yuste J, Wilson RJ, Hosie A, Ogunniyi AD, Paton JC, Thomas G, Brown JS. The effects of methionine acquisition and synthesis on Streptococcus pneumoniae growth and virulence. PLOS ONE. 2013;8:e49638. doi: 10.1371/journal.pone.0049638. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 32170110/National Natural Science Foundation of China
- grant no. 22JCYBJC01060/Natural Science Foundation of Tianjin Municipality
- 63241588/Fundamental Research Funds for the Central Universities
- 32270191/National Natural Science Foundation of China
- 63243161/Fundamental Research Funds for the Central Universities
LinkOut - more resources
Full Text Sources

