Mesenchymal Stromal Cell Secretome and Its Key Bioactive Metabolites Induce Long-Term Neuroprotection After Traumatic Brain Injury in Mice
- PMID: 40536110
- PMCID: PMC12362754
- DOI: 10.1002/advs.202415508
Mesenchymal Stromal Cell Secretome and Its Key Bioactive Metabolites Induce Long-Term Neuroprotection After Traumatic Brain Injury in Mice
Abstract
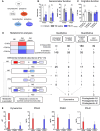
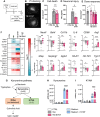
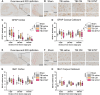
The severe and long-term consequences of traumatic brain injury (TBI) highlight the urgent need for effective neuroprotective therapies. Mesenchymal stromal cells (MSCs) show promise in TBI treatment through their secretome (conditioned media, CM). A low-molecular-weight (<700 Da) CM fraction with neuroprotective effects comparable to total CM after acute brain injury in vitro is previously identified. Here, it is aimed at identifying key bioactive factors, reconstituting them into a synthetic cocktail (SYNT), and evaluating its efficacy in TBI models. Metabolomic profiling identified three prostaglandins and kynurenine, which are used to create SYNT. The SYNT formulation reduced cell death, neuronal damage, and induced protective gene expression changes associated with neuronal protection and microglia modulation toward beneficial phenotype after TBI in vitro. In vivo, SYNT conferred similar long-term functional benefits as CM, improving sensorimotor function up to 6 months and memory preservation at 4 months compared to saline-treated animals, though only CM reduced contusion volume at 5 months. Both treatments modulated neuroinflammation, evidenced by reduced microglial activation and astrogliosis in the pericontusional tissue at 6 months. These findings demonstrate the neuroprotective effects of MSC-secretome treatment in TBI and highlight prostaglandins and kynurenine as key mediators of this response. The findings lay the groundwork for developing a standardized, cell-free therapeutic strategy for TBI based on MSC derivatives.
Keywords: immunomodulation; mesenchymal stromal cells; metabolomics; neuroprotection; secretome; traumatic brain injury.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Maas A. I. R., Menon D. K., Manley G. T., Abrams M., Åkerlund C., Andelic N., Aries M., Bashford T., Bell M. J., Bodien Y. G., Brett B. L., Büki A., Chesnut R. M., Citerio G., Clark D., Clasby B., Cooper D. J., Czeiter E., Czosnyka M., Dams‐O'Connor K., De Keyser V., Diaz‐Arrastia R., Ercole A., van Essen T. A., Falvey É., Ferguson A. R., Figaji A., Fitzgerald M., Foreman B., Gantner D., et al., Lancet Neurol. 2022, 21, 1004.
-
- Pischiutta F., Caruso E., Cavaleiro H., Salgado A. J., Loane D. J., Zanier E. R., Exp. Neurol. 2022, 357, 114199. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
