Down syndrome with Alzheimer's disease brains have increased iron and associated lipid peroxidation consistent with ferroptosis
- PMID: 40536124
- PMCID: PMC12177674
- DOI: 10.1002/alz.70322
Down syndrome with Alzheimer's disease brains have increased iron and associated lipid peroxidation consistent with ferroptosis
Abstract
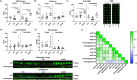
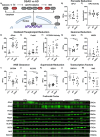
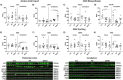
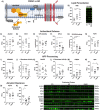
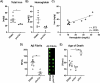
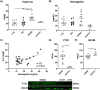
Introduction: Cerebral microbleeds (MBs) are associated with sporadic Alzheimer's disease (AD) and Down syndrome with AD (DSAD). Higher MB iron may cause iron-mediated lipid peroxidation. We hypothesize that amyloid deposition is linked to MB iron and that amyloid precursor protein (APP) triplication increases iron load and lipid peroxidation.
Methods: Prefrontal cortex and cerebellum of cognitively normal control (CTL), AD, and DSAD ApoE3,3 carriers were examined for proteins that mediated iron metabolism, antioxidant response, and amyloid processing in lipid rafts.
Results: Iron was twofold higher in DSAD than in CTL and AD. Iron storage proteins and lipid peroxidation were increased in the prefrontal cortex. The glutathione synthesis protein GCLM was decreased by 50% in both AD and DSAD. Activity of lipid raft GPx4, responsible for membrane repair, was decreased by at least 30% in AD and DSAD.
Discussion: DSAD shows greater lipid peroxidation than AD, consistent with greater MBs and iron load.
Highlights: DSAD has increased ferroptotic-related changes compared to sporadic AD. Lipid rafts that process APP have a loss of protective antioxidant enzymes. Partial and mosaic trisomy lowers the amyloid and iron burden.
Keywords: APP; GCLM; GPx4; GSH; HNE; amyloid; lipid raft; mosaic trisomy; partial trisomy.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the Supporting Information.
Figures








Update of
-
Down syndrome with Alzheimer's disease brains have increased iron and associated lipid peroxidation consistent with ferroptosis.bioRxiv [Preprint]. 2025 Feb 6:2025.02.05.636731. doi: 10.1101/2025.02.05.636731. bioRxiv. 2025. Update in: Alzheimers Dement. 2025 Jun;21(6):e70322. doi: 10.1002/alz.70322. PMID: 39975068 Free PMC article. Updated. Preprint.
Similar articles
-
Down syndrome with Alzheimer's disease brains have increased iron and associated lipid peroxidation consistent with ferroptosis.bioRxiv [Preprint]. 2025 Feb 6:2025.02.05.636731. doi: 10.1101/2025.02.05.636731. bioRxiv. 2025. Update in: Alzheimers Dement. 2025 Jun;21(6):e70322. doi: 10.1002/alz.70322. PMID: 39975068 Free PMC article. Updated. Preprint.
-
Amyloid-β peptide signature associated with cerebral amyloid angiopathy in familial Alzheimer's disease with APPdup and Down syndrome.Acta Neuropathol. 2024 Jul 18;148(1):8. doi: 10.1007/s00401-024-02756-4. Acta Neuropathol. 2024. PMID: 39026031 Free PMC article.
-
Apolipoprotein E abundance is elevated in the brains of individuals with Down syndrome-Alzheimer's disease.Acta Neuropathol. 2025 May 19;149(1):49. doi: 10.1007/s00401-025-02889-0. Acta Neuropathol. 2025. PMID: 40387921 Free PMC article.
-
Resistance and resilience to Alzheimer's disease in Down syndrome.Alzheimers Dement. 2025 Apr;21(4):e70151. doi: 10.1002/alz.70151. Alzheimers Dement. 2025. PMID: 40289889 Free PMC article. Review.
-
Genetically determined Alzheimer's disease research advances: The Down Syndrome & Autosomal Dominant Alzheimer's Disease 2024 Conference.Alzheimers Dement. 2025 Jul;21(7):e70309. doi: 10.1002/alz.70309. Alzheimers Dement. 2025. PMID: 40604347 Free PMC article. Review.
Cited by
-
Air pollution decreases postsynaptic PSD-95 and NMDA receptor subunits in synaptosomes from mouse cerebral cortex.Environ Pollut. 2025 Jul 16:126845. doi: 10.1016/j.envpol.2025.126845. Online ahead of print. Environ Pollut. 2025. PMID: 40681078
-
Age-related declines in mitochondrial Prdx6 contribute to dysregulated muscle bioenergetics.Redox Biol. 2025 Aug 5;86:103808. doi: 10.1016/j.redox.2025.103808. Online ahead of print. Redox Biol. 2025. PMID: 40774144 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- T32-AG000037/National Institutes of Health (NIH)/NIA
- P01-AG055367/National Institutes of Health (NIH)/NIA
- 2022-A-010-SUP/Larry L. Hillblom Foundation
- P50 AG005142/AG/NIA NIH HHS/United States
- R01 AG079806/AG/NIA NIH HHS/United States
- R01-AG051521/National Institutes of Health (NIH)/NIA
- SF811217/Simons Collaboration on Plasticity in the Aging Brain
- R01AG079806/National Institutes of Health (NIH)/NIA
- Navigage Foundation Award
- U01 AG006781/AG/NIA NIH HHS/United States
- Glenn Foundation for Medical Research
- P30-AG066509/National Institutes of Health (NIH)/NIA
- P50-AG005142/National Institutes of Health (NIH)/NIA
- T32 AG000037/AG/NIA NIH HHS/United States
- P50-AG05142/National Institutes of Health (NIH)/NIA
- RF1 AG051521/AG/NIA NIH HHS/United States
- T32 AG052374/AG/NIA NIH HHS/United States
- P01 AG055367/AG/NIA NIH HHS/United States
- P30-AG066530/National Institutes of Health (NIH)/NIA
- P30 AG066519/AG/NIA NIH HHS/United States
- U01-AG006781/National Institutes of Health (NIH)/NIA
- P30 AG066530/AG/NIA NIH HHS/United States
- P30-AG066519/National Institutes of Health (NIH)/NIA
- R01AG079806-02S1/National Institutes of Health (NIH)/NIA
- P30 AG066509/AG/NIA NIH HHS/United States
- T32AG052374/National Institutes of Health (NIH)/NIA
- Cure Alzheimer's Fund
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

