Revolution of AAV in Drug Discovery: From Delivery System to Clinical Application
- PMID: 40536197
- PMCID: PMC12178111
- DOI: 10.1002/jmv.70447
Revolution of AAV in Drug Discovery: From Delivery System to Clinical Application
Abstract
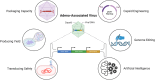
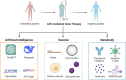
Adeno-associated virus (AAV) is a non-enveloped DNA virus infecting a wide variety of species, tissues, and cell types, which is recognized as a safe and effective method for delivering therapeutic transgenes. AAV vector is the most popular viral gene delivery system in clinical delivery systems with unique and multiple advantages, such as tissue tropism, transduction specificity, long-lasting gene expression, low immune responses, and without host chromosome incorporation. Till now, four AAV-based gene therapy drugs have already been approved by the US Food and Drug Administration (FDA) or European Medicines Agency (EMA). Despite the success of AAV vectors, there are still some remaining challenges that limit further usage, such as poor packaging capacity, low organ specificity, pre-existing humoral immunity, and vector dose-dependent toxicity. In the present review, we address the different approaches to optimize AAV vector delivery system with a focus on capsid engineering, packaging capacity, and immune response at the clinical level. The review further investigates the potential of manipulating AAV vectors in preclinical applications and clinical translation, which emphasizes the challenges and prospects in viral vector selection, drug delivery strategies, immune reactions in cancer, neurodegenerative disease, retinal disease, SARS-CoV-2, and monkeypox. Finally, it forecasts future directions and potential challenges of artificial intelligence (AI), vaccines, and nanobodies, which emphasizes the need for ethical and secure approaches in AAV application.
Keywords: AAV; clinical application; delivery system; drug discovery.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Figures








References
-
- Bartel M. A., Weinstein J. R., and Schaffer D. V., “Directed Evolution of Novel Adeno‐Associated Viruses for Therapeutic Gene Delivery,” Gene Therapy 19 (2012): 694–700. - PubMed
-
- Weitzman M. D. and Linden R. M., “Adeno‐Associated Virus Biology,” Methods in Molecular Biology 807 (2011): 1–23. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

