The present and future of precision oncology and tumor-agnostic therapeutic approaches
- PMID: 40536268
- PMCID: PMC12204399
- DOI: 10.1093/oncolo/oyaf152
The present and future of precision oncology and tumor-agnostic therapeutic approaches
Abstract
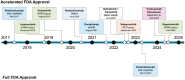
Precision oncology has transformed the treatment landscape for patients with advanced solid tumors. Tumor-agnostic therapies, those that have been approved based on genetic mutations or biomarkers across tumor histology types, are important examples of how the implementation of precision oncology can expand therapeutic options for patients, especially those with rare cancer types and treatment-refractory disease. In this review, we first discuss how advances in next-generation sequencing and molecular profiling have enabled the identification of shared actionable alterations. Subsequently, we explore the current landscape of tumor-agnostic therapies that have received approval from the Food and Drug Administration. We discuss the strengths and limitations of these therapies and evaluate the clinical trial data leading to their approval. In addition, we detail updated results from these clinical trials and additional observational studies reported after the approval. Several factors such as tumor histology, specific alteration types, and the presence of co-alterations are associated with the efficacy of these therapies. Also, challenges remain in understanding resistance mechanisms and predicting response. Looking ahead, we discuss how improved diagnostic tools, novel experimental strategies, and innovative trial designs may further advance the field of precision oncology and improve therapeutic options for patients.
Keywords: biomarker-driven treatment; molecular profiling; precision oncology; targeted therapy; tumor-agnostic therapy.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
Funda Meric-Bernstam reports grant/research support from Jazz Pharmaceuticals, Zymeworks, Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., CCSG, CTSA, Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc., and Taiho Pharmaceutical Co.; consulting fees from AstraZeneca Pharmaceuticals, Becton Dickinson, Calibr (a division of Scripps Research), Daiichi Sankyo, Dava Oncology, Debiopharm, EcoR1 Capital, eFFECTOR Therapeutics, Elevation Oncology, Exelixis, GT Aperion, Incyte, Jazz Pharmaceuticals, LegoChem Biosciences, Lengo Therapeutics, Menarini Group, Molecular Templates, Protai Bio, Ribometrix, Tallac Therapeutics, Tempus, and Zymeworks; honoraria from Dava Oncology; travel support from European Organisation for Research and Treatment of Cancer (EORTC), European Society for Medical Oncology (ESMO), Cholangiocarcinoma Foundation, and Dava Oncology; and advisory committee with Cybrexa, FogPharma, go Therapeutics, Guardant Health, Harbinger Health, Karyopharm Therapeutics, Kivu Biosciences LOXO-Oncology, Mersana Therapeutics, OnCusp Therapeutics, Sanofi Pharmaceuticals, Seagen, Theratechnologies, Zentalis Pharmaceuticals.
Figures
References
-
- Siegel RL, Giaquinto AN, Jemal A.. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. https://doi.org/ 10.3322/caac.21820 - DOI - PubMed
-
- Bailey MH, Tokheim C, Porta-Pardo E, et al. ; MC3 Working Group. Comprehensive characterization of cancer driver genes and mutations. Cell. 2018;173:371-385.e18. https://doi.org/ 10.1016/j.cell.2018.02.060 - DOI - PMC - PubMed
-
- Gao Q, Liang W-W, Foltz SM, et al. ; Fusion Analysis Working Group. Driver fusions and their implications in the development and treatment of human cancers. Cell Rep. 2018;23:227-238.e3. https://doi.org/ 10.1016/j.celrep.2018.03.050 - DOI - PMC - PubMed
-
- Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022;12:31-46. https://doi.org/ 10.1158/2159-8290.CD-21-1059 - DOI - PubMed
-
- Meric-Bernstam F, Johnson A, Holla V, et al. A decision support framework for genomically informed investigational cancer therapy. J Natl Cancer Inst. 2015;107:djv098. https://doi.org/ 10.1093/jnci/djv098 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical