Diagnostic accuracy of real-time PCR assay 'Quantiplus® MTB FAST' for detection of adult pulmonary tuberculosis (PTB): A multi-centric study
- PMID: 40536373
- PMCID: PMC12178193
- DOI: 10.25259/IJMR_767_2025
Diagnostic accuracy of real-time PCR assay 'Quantiplus® MTB FAST' for detection of adult pulmonary tuberculosis (PTB): A multi-centric study
Abstract
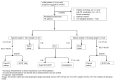
Background & objectives The global target set by the United Nations (UN) high-level meeting on Tuberculosis (TB) for coverage of rapid molecular tests is 100 per cent by 2027. Rapid, affordable molecular tests for early detection of TB are the need of the hour. This study aimed to evaluate the diagnostic accuracy of an open real-time PCR (RT-PCR) assay, Quantiplus®, with reference to Mycobacteria Growth Indicator Tube (MGIT) liquid culture. Methods We conducted a prospective multi-centric diagnostic accuracy study of Quantiplus® assay (version 2.0) at three sites in India for the detection of pulmonary TB in sputum with culture as the reference standard, compared with Xpert® MTB/RIF. A total of 657 adults (>18 yr) with presumptive TB were enrolled consecutively. The Quantiplus® assay uses an extraction-free, quick-lysis protocol and three gene targets for RT-PCR. Results Of the 644 samples analysed, 37 per cent were culture-positive and 32 per cent were smear-positive. The sensitivity and specificity of Quantiplus® assay with reference to MGIT culture were 86 per cent [95% confidence interval (CI): 81-90] and 96 per cent (95% CI: 94-98), respectively, at Ct ≤ 38. The positive and negative predictive values (PPV/NPV) were 93 per cent (95% CI: 89-96%) and 92 per cent (95% CI: 89-94%), respectively. Among the 73 smear-negative culture-positive specimens, the sensitivity and specificity were 61.6 per cent (95% CI: 50-73) and 97 per cent (95% CI: 92-98.6), respectively. The performance of Quantiplus® assay(v2.0) was comparable to Xpert MTB/RIF® (κ=0.83, SE=0.02) at Ct ≤38. Interpretation & conclusions The flexibility of the open RT-PCR assay to be used in any RT-PCR machine makes it a very low-cost (<2 US$) alternative to the expensive cartridge-based tests. This is the first report of validation of an open system RT-PCR assay for the detection of pulmonary TB.
Keywords: Diagnosis; low-cost; molecular test; open RT-PCR; presumptive TB; pulmonary tuberculosis.
Conflict of interest statement
None.
Figures
References
-
- World Health Organization Global tuberculosis report 2024. Available from: https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-heal..., accessed on April 28, 2025.
-
- National Health Mission. Central TB Division, Ministry of Health and Family Welfare, Government of India National TB Elimination Program. India TB report 2024. Available from: https://tbcindia.mohfw.gov.in/wp-content/uploads/2024/10/TB-Report_for-W..., accessed on April 28, 2025.
-
- ICMR Bioethics Unit, Indian Council of Medical Research National Ethical Guidelines for Biomedical and Health Research Involving Human Participants. Available from: https://ethics.ncdirindia.org/asset/pdf/ICMR_National_Ethical_Guidelines..., accessed on April 28, 2025.
-
- ICMR Bioethics Unit, Indian Council of Medical Research Guidance on ethical requirements for laboratory validation testing. Available from: https://www.icmr.gov.in/icmrobject/uploads/Documents/1724842064_guidance..., accessed on April 28, 2025.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources