The Early Detection, Diagnostic Evaluation, and Local Treatment of Prostate Cancer: A Paradigm Shift
- PMID: 40536418
- PMCID: PMC12580837
- DOI: 10.3238/arztebl.m2025.0099
The Early Detection, Diagnostic Evaluation, and Local Treatment of Prostate Cancer: A Paradigm Shift
Abstract
Background: Approximately 75 000 men receive a diagnosis of prostate cancer in Germany each year. New data on the early detection, diagnostic evaluation, and treatment of prostate cancer provide the basis for a paradigm shift in the management of locally confined prostate cancer.
Methods: This narrative review is based on the systematic literature search that was carried out for the 2025 update of the German clinical practice guideline on prostate cancer.
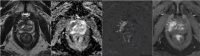
Results: Risk-adapted early detection is now recommended. This involves the measurement of a baseline PSA value at age 45 whose magnitude determines the interval of follow-up testing: once every 5 years for baseline values below 1.5 ng/mL, and once every two years for baseline values between 1.5 and 3 ng/mL. Patients with PSA levels above 3 ng/mL should undergo a repeat PSA test and, if these levels are confirmed, receive a urological risk assessment including prostatic volume, family history, and past medical history. High risk patients should undergo magnetic resonance imaging (MRI) and, if necessary, prostate biopsy. This new PSA-MRI algorithm increases accuracy in detecting clinically significant prostate cancers, enabling the previously recommended annual testing and digital rectal examination to be avoided. Another novelty is that the indication for an active surveillance strategy for men with low-risk prostate cancer has been expanded to ISUP grade group 1 and 2 cancers with favorable risk.
Conclusion: The need for high-quality diagnostic testing, including MRI, with broad geographic coverage will be a major challenge to the health care system, especially with regard to accessibility. Patients can be expected to benefit greatly from the new PSA-MRI algorithm, as it eliminates unnecessary diagnostic testing and treatment while enabling necessary treatment to be initiated earlier and therefore with fewer side effects.
Figures


References
-
- RKI; Krebsregisterdaten Zf, editors. Robert Koch Institut; 2022. Krebs in Deutschland.
-
- de Vos II, Meertens A, Hogenhout R, Remmers S, Roobol MJ. ERSPC Rotterdam Study Group: A detailed evaluation of the effect of prostate-specific antigen-based screening on morbidity and mortality of prostate cancer: 21-year follow-up results of the Rotterdam section of the European Randomised Study of Screening for Prostate Cancer. Eur Urol. 2023;85:426–434. - PubMed
-
- Arsov C, Albers P, Herkommer K, et al. A randomized trial of risk-adapted screening for prostate cancer in young men—results of the first screening round of the PROBASE trial. Int J Cancer. 2022;150:1861–1869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

