Resmetirom-eligible population among US adults: An estimation analysis based on NHANES 2017-March 2020
- PMID: 40536500
- PMCID: PMC12180822
- DOI: 10.1097/HC9.0000000000000755
Resmetirom-eligible population among US adults: An estimation analysis based on NHANES 2017-March 2020
Abstract
Background: Resmetirom received FDA approval for treating adults with noncirrhotic metabolic dysfunction-associated steatohepatitis (MASH) and moderate-to-advanced liver fibrosis (stages F2-F3). Here, we sought to estimate the eligible U.S. adult population for resmetirom therapy, with secondary analysis focusing on individuals with type 2 diabetes mellitus (T2DM).
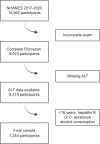
Methods: A cross-sectional analysis was conducted using data from the National Health and Nutrition Examination Survey (NHANES) 2017-March 2020 cycle. Two eligibility scenarios were examined: a liberal scenario requiring ALT >17 U/L for women or >20 U/L for men, controlled attenuation parameter (CAP) >280 dB/m, and liver stiffness measurement (LSM) >8 kPa; and a restrictive scenario requiring ALT >30 U/L for both sexes, CAP >280 dB/m, and LSM >10 kPa. The analysis incorporated sampling weights to generate nationally representative estimates.
Results: The study cohort included 7244 adults (mean age 49.08 y, 49.9% male) with a mean BMI of 29.61 kg/m², mean CAP 263.35 dB/m, and mean LSM 5.8 kPa. An estimated 8.3 million (95% CI: 6.6-9.9 million) adults met the liberal eligibility criteria, while 2.3 million (95% CI: 1.4-3.2 million) met the restrictive criteria. Patients meeting restrictive criteria were predominantly male (76.2% vs. 59.9%) and younger (mean age 46.7 vs. 48.3 y), with similar BMI (38.6 vs. 38.1 kg/m²). Among adults with T2DM, 3.5 million (95% CI: 2.4-4.5 million; 12.2%) met liberal, whereas 0.85 million (95% CI: 0.5-1.2 million; 3.0%) restrictive criteria.
Conclusions: A substantial proportion of U.S. adults meet eligibility criteria for resmetirom treatment, with estimates varying significantly based on the stringency of selection criteria.
Keywords: fibrosis; metabolic dysfunction–associated steatohepatitis; metabolic dysfunction–associated steatotic liver disease; noninvasive test; resmetirom.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Naim Alkhouri has received grant/research support from 89bio, Akero Therapeutics, Arbutus Biopharma, AstraZeneca, BioAge, Boehringer Ingelheim, Bristol Myers Squibb, Corcept Therapeutics, CymaBay Therapeutics, DSM, Galectin Therapeutics, Genentech, Genfit, Gilead Sciences, Healio, Hepagene Therapeutics, Intercept Pharmaceuticals, Inventiva Pharma, Ionis Pharmaceuticals, Ipsen, Lilly, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Noom, NorthSea Therapeutics, Novo Nordisk, Perspectum, Pfizer, PharmaIN, Poxel, Viking Therapeutics, and Zydus Pharmaceuticals; reports speaker’s fees from AbbVie, AstraZeneca, Echosens, Gilead Sciences, Intercept Pharmaceuticals, Ipsen, Madrigal Pharmaceuticals, and Perspectum; and reports consulting for 89bio, Akero, Boehringer Ingelheim, Echosens, Fibronostics, Gilead Sciences, Intercept Pharmaceuticals, Ipsen, Madrigal Pharmaceuticals, NorthSea Therapeutics, Novo Nordisk, Perspectum, Pfizer, and Zydus Pharmaceuticals. Yusuf Yilmaz has served as a consultant to Novo Nordisk, Zydus, Cymabay, Akero, and Echosens. The remaining authors [Eda Kaya, Phuc Le, and Anh Phan] have no conflicts to report.
Figures
References
-
- Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966–1986. - PubMed
-
- Younossi ZM, Paik JM, Stepanova M, Ong J, Alqahtani S, Henry L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J Hepatol. 2024;80:694–701. - PubMed
-
- Paik JM, Kabbara K, Eberly KE, Younossi Y, Henry L, Younossi ZM. Global burden of NAFLD and chronic liver disease among adolescents and young adults. Hepatol. 2022;75:1204–1217. - PubMed
-
- Younossi ZM, Golabi P, Price JK, Owrangi S, Gundu-Rao N, Satchi R, et al. The global epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2024;22:1999–2010.e8. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous