Semaglutide versus other GLP-1 receptor agonists in patients with MASLD
- PMID: 40536520
- PMCID: PMC12180814
- DOI: 10.1097/HC9.0000000000000747
Semaglutide versus other GLP-1 receptor agonists in patients with MASLD
Abstract
Objectives: Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most prevalent chronic liver disease worldwide. While glucagon-like peptide-1 receptor agonists (GLP-1RAs) show promise in MASLD treatment, the comparative effectiveness of semaglutide versus other GLP-1RAs remains unclear. This study aimed to compare clinical outcomes between semaglutide and other GLP-1RAs in patients with MASLD.
Methods: Using the TriNetX Research Network database, we conducted a retrospective cohort study of patients with MASLD newly prescribed GLP-1RAs between December 2017 and September 2023. The primary outcome was a composite of all-cause mortality, major adverse cardiovascular events, major adverse kidney events, and major adverse liver outcomes. Secondary outcomes included the individual components of the primary outcome.
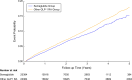
Results: After propensity score matching, 20,384 patients were included in each group. Compared to other GLP-1RAs, semaglutide was associated with a 14% lower risk of primary composite outcomes (31.8 vs. 36.6 events per 10,000 person-years; adjusted HR, 0.86; 95% CI: 0.80-0.93). Semaglutide users showed significantly reduced risks of all-cause mortality (aHR, 0.68; 95% CI: 0.59-0.80) and major adverse liver outcomes (aHR, 0.79; 95% CI: 0.66-0.94). Benefits were consistent across subgroups, including age, sex, obesity status, and diabetes status. Comparative analyses showed superior outcomes with semaglutide versus dulaglutide (aHR, 0.88; 95% CI: 0.81-0.96) and liraglutide (aHR, 0.83; 95% CI: 0.71-0.97).
Conclusions: In patients with MASLD, semaglutide use was associated with significantly better clinical outcomes compared to other GLP-1RAs, particularly in reducing mortality and major adverse liver outcome risks. These findings suggest semaglutide may be the preferred GLP-1RA choice for MASLD treatment.
Keywords: glucagon-like peptide-1 receptor agonists; metabolic dysfunction–associated steatotic liver disease; real-world evidence; semaglutide.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors have no conflicts to report.
Figures


References
-
- Tacke F, Horn P, Wai-Sun Wong V, Ratziu V, Bugianesi E, Francque S, et al. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492–542. - PubMed
-
- Younossi ZM, Wong G, Anstee QM, Henry L. The global burden of liver disease. Clin Gastroenterol Hepatol. 2023;21:1978–1991. - PubMed
-
- Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J Hepatol. 2019;71:212–221. - PubMed
-
- Wong VW, Ekstedt M, Wong GL, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79:842–852. - PubMed
-
- Dong X, Li JM, Lu XL, Lin XY, Hong MZ, Weng S, et al. Global burden of adult non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) has been steadily increasing over the past decades and is expected to persist in the future. Transl Gastroenterol Hepatol. 2024;9:33. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

