Cenobamate for Adjunctive Treatment in Adult and Pediatric Patients with Refractory Lennox-Gastaut Syndrome: A Retrospective Chart Review
- PMID: 40536598
- PMCID: PMC12255617
- DOI: 10.1007/s40120-025-00779-x
Cenobamate for Adjunctive Treatment in Adult and Pediatric Patients with Refractory Lennox-Gastaut Syndrome: A Retrospective Chart Review
Abstract
Introduction: Lennox-Gastaut syndrome (LGS) is a particularly severe developmental epileptic encephalopathy (DEE) characterized by multiple types of drug-resistant, incapacitating seizures. Despite aggressive therapy including polypharmacy, surgery, implanted devices, and dietary therapy, the prognosis remains poor, with frequent ongoing seizures and risk of injury and early death. Cenobamate (CNB) is an antiseizure medication (ASM) approved for the treatment of focal seizures in adults, but real-world experience in patients with DEEs has shown promising reductions in seizure frequency.
Methods: This retrospective chart review determined the effectiveness and tolerability of CNB in 36 adult and pediatric patients with LGS under the treatment of one physician.
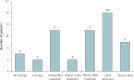
Results: Among 36 patients (69% male, median age 15.5 years) with LGS, 86% experienced a reduction in seizure frequency after the addition of CNB (median treatment duration 23 months), including ≥ 75% reduction in 22 patients (61%) and seizure freedom in 5 patients (14%). A substantial proportion of patients (75%, n = 27) successfully reduced their concomitant medications, including the lowering or discontinuation of cannabidiol in 19 patients and clobazam in 21 patients. Adverse events were reported in two-thirds of patients, reflecting the same symptoms reported in the original approval trials, with somnolence being the most common.
Conclusions: This chart review provides promising evidence for the efficacy of CNB in treating LGS. Additional prospective studies will help to clarify CNB's efficacy and safety profile for patients with LGS.
Keywords: Cenobamate; Lennox–Gastaut syndrome; Pharmacotherapy; Refractory epilepsy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of Interest: Karen Keough: Speaker, SK Life Science, Inc. Affiliation at time of study: Pediatrix Medical Group. Current affiliation: Child Neurology and Consultants of Austin. Alec Romick: Nothing to disclose. Ethical Approval: The study was determined to be exempt from IRB requirements by WCG IRB (Puyallup, WA, USA). The authors confirm that they have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
Similar articles
-
Cenobamate in developmental and epileptic encephalopathies and generalized epilepsies: A case report on epilepsy with myoclonic-atonic seizures and systematic review of current evidence.Seizure. 2025 Jul;129:1-8. doi: 10.1016/j.seizure.2025.03.012. Epub 2025 Mar 15. Seizure. 2025. PMID: 40138943
-
Real-world effectiveness and tolerability of cenobamate in drug-resistant epilepsy: A retrospective analysis of the patients included into the Early Access Programs (EAP) in Germany, France, and United Kingdom.Epilepsia Open. 2025 Jun;10(3):736-748. doi: 10.1002/epi4.70021. Epub 2025 Mar 22. Epilepsia Open. 2025. PMID: 40119878 Free PMC article.
-
Real-world utilization of Cenobamate as adjunct therapy in office-based neurology: practical tips and insights for titration.Front Neurol. 2025 Jun 13;16:1558614. doi: 10.3389/fneur.2025.1558614. eCollection 2025. Front Neurol. 2025. PMID: 40584530 Free PMC article.
-
Rufinamide add-on therapy for refractory epilepsy.Cochrane Database Syst Rev. 2018 Apr 25;4(4):CD011772. doi: 10.1002/14651858.CD011772.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Nov 8;11:CD011772. doi: 10.1002/14651858.CD011772.pub3. PMID: 29691835 Free PMC article. Updated.
-
Efficacy and safety of six new antiseizure medications for adjunctive treatment of focal epilepsy and epileptic syndrome: A systematic review and network meta-analysis.Epilepsy Behav. 2024 Mar;152:109653. doi: 10.1016/j.yebeh.2024.109653. Epub 2024 Jan 25. Epilepsy Behav. 2024. PMID: 38277848
References
LinkOut - more resources
Full Text Sources