Lactobacillus johnsonii HL79 mitigate plateau environment-induced hippocampal dysfunction in mice
- PMID: 40536601
- PMCID: PMC12179028
- DOI: 10.1186/s13568-025-01898-2
Lactobacillus johnsonii HL79 mitigate plateau environment-induced hippocampal dysfunction in mice
Abstract
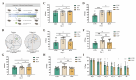
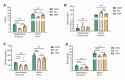
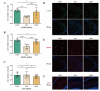
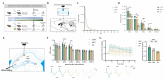
Plateau environment represents a common terrestrial characterized by multistress conditions including hypobaric hypoxia, low temperature, and intense radiation, yet sustain over 100 million permanent or transient inhabitants. While this extreme environment exerts profound impacts on cerebral architecture and gut microbiota homeostasis, precipitating cognitive deficits and microbiome-derived intestinal pathologies, the mechanistic interplay between plateau environment adaptation and microbial dynamics remains contentious. Here, we employ a microbiota-gut-brain axis framework to investigate whether probiotic intervention can ameliorate hippocampal impairments induced by simulated plateau environment exposure (3500-4000 m) in mice. Through simulated plateau environment exposure experiments, we revealed that extreme high-altitude conditions induced hippocampal memory dysfunction in mice, exacerbated oxidative stress damage in hippocampal tissues, and altered synaptic plasticity-related biomarkers including CREB transcription factor, BDNF protein levels, and electrophysiological power spectra. Administration of HL79 alleviated these burdens, including memory dysfunction and tissue damage, though complete reversal was not achieved. Combined hippocampal transcriptomic analyses suggested that HL79's beneficial effects primarily involved modulation of lipid-related gene expression in the hippocampus, consistent with prior reports of plateau environmental impacts on gene expression. Serum metabolomic results further reinforced this inference that differential metabolites regulated by HL79 are mainly enriched in bile secretion, taurine and hypotaurine metabolism, linoleic acid metabolism, and PPAR signaling pathways, though the precise regulatory mechanisms require further elucidation. This research provides a novel microbiota-gut-brain axis-based regulatory strategy for adaptation to extreme plateau environments and offers new evidence for understanding the relationship between gut microbiota and plateau environment adaptation at high elevations.
Keywords: Microbiota-gut-brain axis; Plateau environment; Probiotics; Spatial memory dysfunction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The experimental protocol was approved by the Research Ethics Committee, College of Veterinary Medicine, Sichuan Agricultural University, China (approval number SYXKchuan2024-0187). Consent for publication: All authors consent to the publication of this work. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Research on the correlation between gut microbiota and brain cognitive function under chronic hypoxia at high altitude.Front Neurosci. 2025 Jun 19;19:1600069. doi: 10.3389/fnins.2025.1600069. eCollection 2025. Front Neurosci. 2025. PMID: 40613089 Free PMC article.
-
Radiation-induced injury and the gut microbiota: insights from a microbial perspective.Therap Adv Gastroenterol. 2025 Jun 16;18:17562848251347347. doi: 10.1177/17562848251347347. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40535532 Free PMC article. Review.
-
Integrating Gut Microbiome and Metabolomics with Magnetic Resonance Enterography to Advance Bowel Damage Prediction in Crohn's Disease.J Inflamm Res. 2025 Jun 11;18:7631-7649. doi: 10.2147/JIR.S524671. eCollection 2025. J Inflamm Res. 2025. PMID: 40535353 Free PMC article.
-
Plateau Environment, Gut Microbiota, and Depression: A Possible Concealed Connection?Curr Issues Mol Biol. 2025 Jun 25;47(7):487. doi: 10.3390/cimb47070487. Curr Issues Mol Biol. 2025. PMID: 40728956 Free PMC article. Review.
-
Lactobacillus johnsonii HL79 modulates the microbiota-gut-brain axis to protect cognitive function in mice chronically exposed to high altitude.Front Microbiol. 2025 Mar 7;16:1561400. doi: 10.3389/fmicb.2025.1561400. eCollection 2025. Front Microbiol. 2025. PMID: 40124891 Free PMC article.
References
-
- Baião R, Capitão LP, Higgins C, Browning M, Harmer CJ, Burnet PWJ (2023) Multispecies probiotic administration reduces emotional salience and improves mood in subjects with moderate depression: a randomised, double-blind, placebo-controlled study. Psychol Med 53(8):3437–3447. 10.1017/S003329172100550X - PMC - PubMed
-
- Benedetti F, Durando J, Giudetti L, Pampallona A, Vighetti S (2015) High-altitude headache: the effects of real vs sham oxygen administration. Pain 156(11):2326–2336. 10.1097/j.pain.0000000000000288 - PubMed
Grants and funding
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- U23A20476/Joint Funds of the National Natural Science Foundation of China
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- LSKJ202309/Key Science and Technology Project of Lhasa, Tibet
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
- 2023ZYJM001/Key Research and Development Project of the Tibet Autonomous Region
LinkOut - more resources
Full Text Sources

